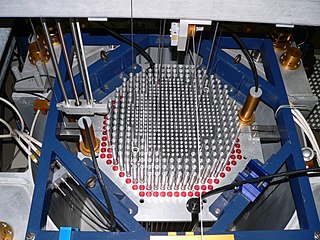
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of 2022, the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world.

Tritium or hydrogen-3 is a rare and radioactive isotope of hydrogen with a half-life of ~12.3 years. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (protium) contains one proton and zero neutrons, and that of hydrogen-2 (deuterium) contains one proton and one neutron.

A nuclear meltdown is a severe nuclear reactor accident that results in core damage from overheating. The term nuclear meltdown is not officially defined by the International Atomic Energy Agency or by the United States Nuclear Regulatory Commission. It has been defined to mean the accidental melting of the core of a nuclear reactor, however, and is in common usage a reference to the core's either complete or partial collapse.
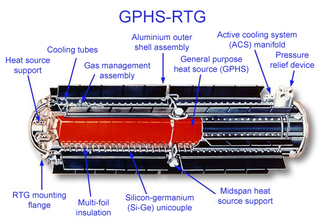
A radioisotope thermoelectric generator, sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioactive material into electricity by the Seebeck effect. This type of generator has no moving parts and is ideal for deployment in remote and harsh environments for extended periods with no risk of parts wearing out or malfunctioning.

A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, large radioactivity release to the environment, reactor core melt." The prime example of a "major nuclear accident" is one in which a reactor core is damaged and significant amounts of radioactive isotopes are released, such as in the Chernobyl disaster in 1986 and Fukushima nuclear disaster in 2011.

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..

The Mayak Production Association is one of the largest nuclear facilities in the Russian Federation, housing a reprocessing plant. The closest settlements are Ozyorsk to the northwest and Novogornyi to the south.
In nuclear engineering, prompt criticality describes a nuclear fission event in which criticality is achieved with prompt neutrons alone and does not rely on delayed neutrons. As a result, prompt supercriticality causes a much more rapid growth in the rate of energy release than other forms of criticality. Nuclear weapons are based on prompt criticality, while nuclear reactors rely on delayed neutrons or external neutrons to achieve criticality.

The Windscale fire of 10 October 1957 was the worst nuclear accident in the United Kingdom's history, and one of the worst in the world, ranked in severity at level 5 out of 7 on the International Nuclear Event Scale. The fire was in Unit 1 of the two-pile Windscale site on the north-west coast of England in Cumberland. The two graphite-moderated reactors, referred to at the time as "piles," had been built as part of the British post-war atomic bomb project. Windscale Pile No. 1 was operational in October 1950, followed by Pile No. 2 in June 1951.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.

The Chernobyl disaster began on 26 April 1986 with the explosion of the No. 4 reactor of the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR, close to the border with the Byelorussian SSR, in the Soviet Union. It is one of only two nuclear energy accidents rated at seven—the maximum severity—on the International Nuclear Event Scale, the other being the 2011 Fukushima nuclear accident in Japan. The initial emergency response and subsequent mitigation efforts involved more than 500,000 personnel and cost an estimated 18 billion roubles—roughly US$68 billion in 2019, adjusted for inflation. It is considered the worst nuclear disaster in history.

Cobalt-60 (60Co) is a synthetic radioactive isotope of cobalt with a half-life of 5.2714 years. It is produced artificially in nuclear reactors. Deliberate industrial production depends on neutron activation of bulk samples of the monoisotopic and mononuclidic cobalt isotope 59
Co
. Measurable quantities are also produced as a by-product of typical nuclear power plant operation and may be detected externally when leaks occur. In the latter case the incidentally produced 60
Co
is largely the result of multiple stages of neutron activation of iron isotopes in the reactor's steel structures via the creation of its 59
Co
precursor. The simplest case of the latter would result from the activation of 58
Fe
. 60
Co
undergoes beta decay to the stable isotope nickel-60. The activated nickel nucleus emits two gamma rays with energies of 1.17 and 1.33 MeV, hence the overall equation of the nuclear reaction is: 59
27Co
+ n → 60
27Co
→ 60
28Ni
+ e− + 2 γ

Caesium-137, cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from spontaneous fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. Caesium-137 has a relatively low boiling point of 671 °C (1,240 °F) and easily becomes volatile when released suddenly at high temperature, as in the case of the Chernobyl nuclear accident and with atomic explosions, and can travel very long distances in the air. After being deposited onto the soil as radioactive fallout, it moves and spreads easily in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. Caesium-137 was discovered by Glenn T. Seaborg and Margaret Melhase.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.

Strontium-90 is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and industry and is an isotope of concern in fallout from nuclear weapons, nuclear weapons testing, and nuclear accidents.
This article compares the radioactivity release and decay from the Chernobyl disaster with various other events which involved a release of uncontrolled radioactivity.

The Kyshtym disaster, sometimes referred to as the Mayak disaster or Ozyorsk disaster in newer sources, was a radioactive contamination accident that occurred on 29 September 1957 at Mayak, a plutonium production site for nuclear weapons and nuclear fuel reprocessing plant located in the closed city of Chelyabinsk-40 in Chelyabinsk Oblast, Russian SFSR, Soviet Union.
The RaLa Experiment, or RaLa, was a series of tests during and after the Manhattan Project designed to study the behavior of converging shock waves to achieve the spherical implosion necessary for compression of the plutonium pit of the nuclear weapon. The experiment used significant amounts of a short-lived radioisotope lanthanum-140, a potent source of gamma radiation; the RaLa is a contraction of Radioactive Lanthanum. The method was proposed by Robert Serber and developed by a team led by the Italian experimental physicist Bruno Rossi.
From 1946 through 1993, thirteen countries used ocean disposal or ocean dumping as a method to dispose of nuclear/radioactive waste with an approximation of 200,000 tons sourcing mainly from the medical, research and nuclear industry.

The Fukushima Daiichi nuclear disaster genshiryoku hatsudensho jiko) was a series of equipment failures, nuclear meltdowns, and releases of radioactive materials at the Fukushima I Nuclear Power Plant, following the Tōhoku earthquake and tsunami on 11 March 2011. It is the largest nuclear disaster since the Chernobyl disaster of 1986.














