Related Research Articles

Exponentiation is a mathematical operation, written as bn, involving two numbers, the baseb and the exponent or powern, and pronounced as "b raised to the power of n". When n is a positive integer, exponentiation corresponds to repeated multiplication of the base: that is, bn is the product of multiplying n bases:

Sterilization refers to any process that removes, kills, or deactivates all forms of life and other biological agents like prions present in a specific surface, object or fluid, for example food or biological culture media. Sterilization can be achieved through various means, including heat, chemicals, irradiation, high pressure, and filtration. Sterilization is distinct from disinfection, sanitization, and pasteurization, in that those methods reduce rather than eliminate all forms of life and biological agents present. After sterilization, an object is referred to as being sterile or aseptic.

Planetary protection is a guiding principle in the design of an interplanetary mission, aiming to prevent biological contamination of both the target celestial body and the Earth in the case of sample-return missions. Planetary protection reflects both the unknown nature of the space environment and the desire of the scientific community to preserve the pristine nature of celestial bodies until they can be studied in detail.
Infection prevention and control is the discipline concerned with preventing healthcare-associated infections; a practical rather than academic sub-discipline of epidemiology. In Northern Europe, infection prevention and control is expanded from healthcare into a component in public health, known as "infection protection". It is an essential part of the infrastructure of health care. Infection control and hospital epidemiology are akin to public health practice, practiced within the confines of a particular health-care delivery system rather than directed at society as a whole.

"F0" is defined as the number of equivalent minutes of steam sterilization at temperature 121.1 °C (250 °F) delivered to a container or unit of product calculated using a z-value of 10 °C. The term F-value or "FTref/z" is defined as the equivalent number of minutes to a certain reference temperature (Tref) for a certain control microorganism with an established Z-value.

Germ-free organisms are multi-cellular organisms that have no microorganisms living in or on them. Such organisms are raised using various methods to control their exposure to viral, bacterial or parasitic agents. When known microbiota are introduced to a germ-free organism, it usually is referred to as a gnotobiotic organism, however technically speaking, germ-free organisms are also gnotobiotic because the status of their microbial community is known. Due to lacking a microbiome, many germ-free organisms exhibit health deficits such as defects in the immune system and difficulties with energy acquisition. Typically germ-free organisms are used in the study of a microbiome where careful control of outside contaminants is required.
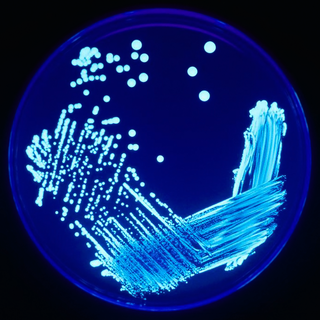
In microbiology, streaking is a technique used to isolate a pure strain from a single species of microorganism, often bacteria. Samples can then be taken from the resulting colonies and a microbiological culture can be grown on a new plate so that the organism can be identified, studied, or tested.
Barrier isolator is a general term that includes two types of devices: isolators and restricted access barriers (RABS). Both are devices that provide a physical and aerodynamic barrier between the external clean room environment and a work process. The isolator design is the more dependable of the two barrier design choices, as it prevents contamination hazards by achieving a more comprehensive separation of the processing environment from the surrounding facility. Nonetheless, both Isolator and RABS designs are contemporary approaches developed over the last 35 years and a great advancement over designs of the 1950s-70s that were far more prone to microbial contamination problems.
Pharmaceutical Microbiology is an applied branch of Microbiology. It involves the study of microorganisms associated with the manufacture of pharmaceuticals e.g. minimizing the number of microorganisms in a process environment, excluding microorganisms and microbial byproducts like exotoxin and endotoxin from water and other starting materials, and ensuring the finished pharmaceutical product is sterile. Other aspects of pharmaceutical microbiology include the research and development of anti-infective agents, the use of microorganisms to detect mutagenic and carcinogenic activity in prospective drugs, and the use of microorganisms in the manufacture of pharmaceutical products like insulin and human growth hormone.
Aseptic processing is a processing technique wherein commercially thermally sterilized liquid products are packaged into previously sterilized containers under sterile conditions to produce shelf-stable products that do not need refrigeration. Aseptic processing has almost completely replaced in-container sterilization of liquid foods, including milk, fruit juices and concentrates, cream, yogurt, salad dressing, liquid egg, and ice cream mix. There has been an increasing popularity for foods that contain small discrete particles, such as cottage cheese, baby foods, tomato products, fruit and vegetables, soups, and rice desserts.
Dry heat sterilization of an article is one of the earliest forms of sterilization practiced. It uses hot air that is either free from water vapor or has very little of it, where this moisture plays a minimal or no role in the process of sterilization.
Moist heat sterilization describes sterilization techniques that use hot water vapor as a sterilizing agent. Heating an article is one of the earliest forms of sterilization practiced. The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules.
In microbiology, in the context of a sterilization procedure, the D-value or decimal reduction time is the time required, at a given condition or set of conditions, to achieve a log reduction, that is, to kill 90% of relevant microorganisms. A D-value is denoted with the capital letter "D". Thus, after an exposure time of 1 D, only 10% of the organisms originally present in a microbial colony would remain. The term originated in assessments of microbes' thermal resistance and in thermal death time analysis; however, it now has analogous uses in other microbial resistance and death rate applications, such as for ethylene oxide and radiation processing.
Bacillus pumilus is a Gram-positive, aerobic, spore-forming bacillus commonly found in soil.
Bioburden is normally defined as the number of bacteria living on a surface that has not been sterilized.
In mathematics, in the field of tropical analysis, the log semiring is the semiring structure on the logarithmic scale, obtained by considering the extended real numbers as logarithms. That is, the operations of addition and multiplication are defined by conjugation: exponentiate the real numbers, obtaining a positive number, add or multiply these numbers with the ordinary algebraic operations on real numbers, and then take the logarithm to reverse the initial exponentiation. Such operations are also known as, e.g., logarithmic addition, etc. As usual in tropical analysis, the operations are denoted by ⊕ and ⊗ to distinguish them from the usual addition + and multiplication ×. These operations depend on the choice of base b for the exponent and logarithm, which corresponds to a scale factor, and are well-defined for any positive base other than 1; using a base b < 1 is equivalent to using a negative sign and using the inverse 1/b > 1. If not qualified, the base is conventionally taken to be e or 1/e, which corresponds to e with a negative.

The central sterile services department (CSSD), also called sterile processing department (SPD), sterile processing, central supply department (CSD), or central supply, is an integrated place in hospitals and other health care facilities that performs sterilization and other actions on medical devices, equipment and consumables; for subsequent use by health workers in the operating theatre of the hospital and also for other aseptic procedures, e.g. catheterization, wound stitching and bandaging in a medical, surgical, maternity or paediatric ward.

An inoculation needle is a laboratory equipment used in the field of microbiology to transfer and inoculate living microorganisms. It is one of the most commonly implicated biological laboratory tools and can be disposable or re-usable. A standard reusable inoculation needle is made from nichrome or platinum wire affixed to a metallic handle. A disposable inoculation needle is often made from plastic resin. The base of the needle is dulled, resulting in a blunted end.
References
- ↑ Agalloco, James; Akers, James; Madsen, Russell (October 2004), Aseptic Processing: A Review of Current Industry Practice, Pharmtech.com
- ↑ Mosley, Gregg (May 2008), "Sterility Assurance Level (SAL) - The term and its definition continues to cause confusion in the industry" (PDF), PMF Newsletter, Pharmaceutical Microbiology Forum, 14 (5), archived from the original (PDF) on November 21, 2008
- ↑ The following simplified summary may be used to explain these concepts in F0 an easily understood manner Archived February 3, 2007, at the Wayback Machine