 | |
| Names | |
|---|---|
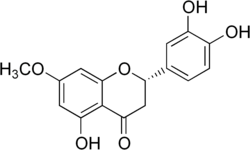
| IUPAC name (2S)-3′,4′,5-Trihydroxy-7-methoxyflavan-4-one | |
| Systematic IUPAC name (2S)-2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names 7-Methoxy-3′,4′,5-trihydroxyflavanone | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.28 g/mol |
| Density | 1.458 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sterubin (7-methoxy-3',4',5-trihydroxyflavanone) is a bitter-masking flavanone extracted from Yerba Santa ( Eriodictyon californicum ) a plant growing in America. [1]
Sterubin is one of the four flavanones identified by Symrise in this plant which elicit taste-modifying properties. The others are homoeriodictyol, its sodium salt, and eriodictyol. [2]
Recent research has demonstrated some neuroprotective properties of Sterubin in vitro, but more research is needed before it can be considered a true drug candidate. [3] [4] [5]