
William Nunn Lipscomb Jr. was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3, the Zundel cation, which is a symmetric dihydrate, H+(H2O)2, and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to its three neighbors.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
Mechanochemistry is the initiation of chemical reactions by mechanical phenomena. Mechanochemistry thus represents a fourth way to cause chemical reactions, complementing thermal reactions in fluids, photochemistry, and electrochemistry. Conventionally mechanochemistry focuses on the transformations of covalent bonds by mechanical force. Not covered by the topic are many phenomena: phase transitions, dynamics of biomolecules, and sonochemistry.
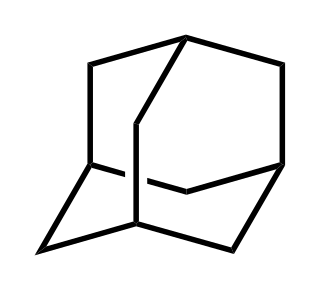
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

A Stone–Wales defect is a crystallographic defect that involves the change of connectivity of two π-bonded carbon atoms, leading to their rotation by 90° with respect to the midpoint of their bond. The reaction commonly involves conversion between a naphthalene-like structure into a fulvalene-like structure, that is, two rings that share an edge vs two separate rings that have vertices bonded to each other.
In chemistry and molecular physics, fluxionalmolecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.

The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry, and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. As such, fluoroalkanes like tetrafluoromethane are some of the most unreactive organic compounds.

In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally in various contexts such as ice, bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2.
Melting-point depression is the phenomenon of reduction of the melting point of a material with a reduction of its size. This phenomenon is very prominent in nanoscale materials, which melt at temperatures hundreds of degrees lower than bulk materials.

James Bernhard Anderson was an American chemist and physicist. From 1995 to 2014 he was Evan Pugh Professor of Chemistry and Physics at the Pennsylvania State University. He specialized in Quantum Chemistry by Monte Carlo methods, molecular dynamics of reactive collisions, kinetics and mechanisms of gas phase reactions, and rare-event theory.

Calcium monohydride is a molecule composed of calcium and hydrogen with formula CaH. It can be found in stars as a gas formed when calcium atoms are present with hydrogen atoms.
Vincenzo Aquilanti is an Italian chemist, emeritus professor at the University of Perugia.
Graphene is the only form of carbon in which every atom is available for chemical reaction from two sides. Atoms at the edges of a graphene sheet have special chemical reactivity. Graphene has the highest ratio of edge atoms of any allotrope. Defects within a sheet increase its chemical reactivity. The onset temperature of reaction between the basal plane of single-layer graphene and oxygen gas is below 260 °C (530 K). Graphene combusts at 350 °C (620 K). Graphene is commonly modified with oxygen- and nitrogen-containing functional groups and analyzed by infrared spectroscopy and X-ray photoelectron spectroscopy. However, determination of structures of graphene with oxygen- and nitrogen- functional groups requires the structures to be well controlled.









