
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cancer, and pancreatic cancer. It is administered by intravenous injection. There is also an albumin-bound formulation.

Taxaceae, commonly called the yew family, is a coniferous family which includes six extant and two extinct genera, and about 30 species of plants, or in older interpretations three genera and 7 to 12 species.
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and anti-inflammatory.

Taxanes are a class of diterpenes. They were originally identified from plants of the genus Taxus (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer.

Taxus canadensis, the Canada yew or Canadian yew, is a conifer native to central and eastern North America, thriving in swampy woods, ravines, riverbanks and on lake shores. Locally called simply "yew", this species is also referred to as American yew or ground-hemlock.

Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel (Taxol). This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew. Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.

Taxus globosa, the Mexican yew, is an evergreen shrub and one of the eight species of yew. The Mexican yew is a rare species, only known to be found in a small number of locations in eastern Mexico, Guatemala, El Salvador and Honduras, and is listed as an endangered species. The Mexican yew is a shrub that grows to an average height of 4.6m. It has large, sharp light green needles growing in ranks on either side of its branches.

A mitotic inhibitor is a drug that inhibits mitosis, or cell division. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow and eventually spread through the body (metastasize) through continuous mitotic division. Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.
In enzymology, a taxane 10beta-hydroxylase (EC 1.14.13.76) is an enzyme that catalyzes the chemical reaction
In enzymology, a taxane 13alpha-hydroxylase (EC 1.14.13.77) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2alpha-hydroxytaxane 2-O-benzoyltransferase is an enzyme that catalyzes the chemical reaction
Taxoids are a class of derivatives from taxol, that is, paclitaxel. They were developed for their anticancer chemotherapeutic properties. Taxoids are usually treated as synonymous with taxanes; for example, a major medical dictionary defines the two terms with the same definition phrasing, and in another the phrasing varies slightly but conveys nearly identical meaning.

Abeotaxanes are a class of taxoid molecules with a core 5/7/6 type ring structure. This structure varies from the 6/8/6 or 6/10/6-membered core ring found in conventional taxoids such as paclitaxel or docetaxel. The core carbon skeleton of a normal natural product taxane has a 6-membered A ring, 8-membered B ring and a 6-membered C ring, combined with conventional side chains. It is the side chains that provide most of the activity for regular taxanes. Abeotaxanes are compounds containing 3 altered ring structures, where ring A is 5 members, ring B is 7 members and ring C is 6 members, combined with conventional side chains.
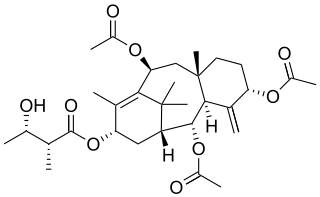
Yunnanxane is a bioactive taxane diterpenoid first isolated from Taxus wallichiana. Yunnanxane was later isolated from cell cultures of Taxus cuspidata and Taxus chinensis. Four homologous esters of yunnanxane have also been isolated from Taxus. Yunnanxane is reported to have anticancer activity in vitro.
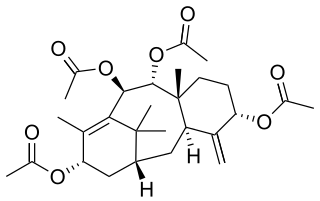
Taxusin is a taxane isolate derived from Taxus wallichiana. Taxusin can be used to synthesize taxadiene -diol and -triol derivatives via deoxygenation.

Decinnamoyltaxinine J is a taxane isolate derived from Taxus brevifolia.

Taxagifine is a taxane isolated from Taxus.

Taxinine M is a tetracyclic taxane isolate derived from Taxus brevifolia, Taxus chinensis, and Taxus mairei.
Taxuyunnanines is a class of taxoids isolated from plants of the genus Taxus.

Taxine alkaloids, which are often named under the collective title of taxines, are the toxic chemicals that can be isolated from the yew tree. The amount of taxine alkaloids depends on the species of yew, with Taxus baccata and Taxus cuspidata containing the most. The major taxine alkaloids are taxine A and taxine B although there are at least 10 different alkaloids. Until 1956, it was believed that all the taxine alkaloids were one single compound named taxine.













