Lead(II) azide Pb(N3)2 is an inorganic compound. More so than other azides, it is explosive. It is used in detonators to initiate secondary explosives. In a commercially usable form, it is a white to buff powder.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
In chemical synthesis, click chemistry is a class of simple, atom-economy reactions commonly used for joining two molecular entities of choice. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, biomimetic and molecular machinery applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.

Copper(II) azide is a medium density explosive with the molecular formula Cu(N3)2.

Diphenylphosphoryl azide (DPPA) is an organic compound. It is widely used as a reagent in the synthesis of other organic compounds.

Morten Peter Meldal is a Danish chemist and Nobel laureate. He is a professor of chemistry at the University of Copenhagen in Copenhagen, Denmark. He is best known for developing the CuAAC-click reaction, concurrently with but independent of Valery V. Fokin and K. Barry Sharpless.
Nitrogen tribromide is a chemical compound with the formula NBr3. It is extremely explosive in its pure form, even at −100 °C, and was not isolated until 1975. It is a deep-red and volatile solid.

n-Propyl azide is an organic compound with the formula CH3CH2CH2N3. A white solid, it is a simple organic azide.

Silicon tetraazide is a thermally unstable binary compound of silicon and nitrogen with a nitrogen content of 85.7%. This high-energy compound combusts spontaneously and can only be studied in a solution. A further coordination to a six-fold coordinated structure such as a hexaazidosilicate ion [Si(N3)6]2− or as an adduct with bicationic ligands Si(N3)4·L2 will result in relatively stable, crystalline solids that can be handled at room temperature.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this chemical.

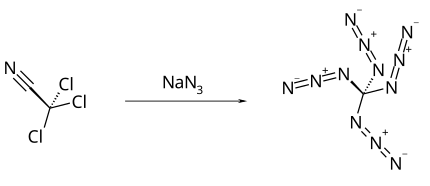
Cyanogen azide, N3CN or CN4, is an azide compound of carbon and nitrogen which is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F. D. Marsh at DuPont in the early 1960s. There had been earlier claims of discovering it as a crystalline solid, which were incorrect.

1-Diazidocarbamoyl-5-azidotetrazole, often jokingly referred to as azidoazide azide, is a heterocyclic inorganic compound with the formula C2N14. It is a highly reactive and extremely sensitive explosive.

Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
Iron(III) azide, also called ferric azide, is a chemical compound with the formula Fe(N3)3. It is an extremely explosive, impact-sensitive, hygroscopic dark brown solid. This compound is used to prepare various azidoalkanes, such as n-butyl azide, from alkenes via formation of alkylboranes and subsequent anti-Markovnikov addition of azide group.
Homoleptic azido compounds are chemical compounds in which the only anion or ligand is the azide group, -N3. The breadth of homoleptic azide compounds spans nearly the entire periodic table. With rare exceptions azido compounds are highly shock sensitive and need to be handled with the upmost caution. Binary azide compounds can take on several different structures including discrete compounds, or one- two, and three-dimensional nets, leading some to dub them as "polyazides". Reactivity studies of azide compounds are relatively limited due to how sensitive they can be. The sensitivity of these compounds tends to be correlated with the amount of ionic or covalent character the azide-element bond has, with ionic character being far more stable than covalent character. Therefore, compounds such as silver or sodium azide – which have strong ionic character – tend to possess more synthetic utility than their covalent counterparts. A few other notable exceptions include polymeric networks which possess unique magnetic properties, group 13 azides which unlike most other azides decompose to nitride compounds (important materials for semiconductors), other limited uses as synthetic reagents for the transfer of azide groups, or for research into high-energy-density matter.