Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C, which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Zinc sulfate describes a family of inorganic compounds with the formula ZnSO4(H2O)x. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O. As early as the 16th century it was prepared on the large scale, and was historically known as "white vitriol" (the name was used, for example, in 1620s by the collective writing under the pseudonym of Basil Valentine). Zinc sulfate and its hydrates are colourless solids.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component.

Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.

Thallium azide, TlN3, is a yellow-brown crystalline solid poorly soluble in water. Although it is not nearly as sensitive to shock or friction as lead azide, it can easily be detonated by a flame or spark. It can be stored safely dry in a closed non-metallic container.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.

Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.
Rubidium hydrogen sulfate, sometimes referred to as rubidium bisulfate, is the half neutralized rubidium salt of sulfuric acid. It has the formula RbHSO4.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. Like most azides, it is explosive.

Caesium oxalate, or dicesium oxalate, or cesium oxalate is a chemical compound with the chemical formula Cs2C2O4. It is a caesium salt of oxalic acid. It consists of caesium cations Cs+ and oxalate anions C2O2−4.

Transition metal azide complexes are coordination complexes containing one or more azide (N3−) ligands.
Cobalt compounds are chemical compounds formed by cobalt with other elements.
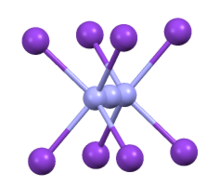
Homoleptic azido compounds are chemical compounds in which the only anion or ligand is the azide group, -N3. The breadth of homoleptic azide compounds spans nearly the entire periodic table. With rare exceptions azido compounds are highly shock sensitive and need to be handled with the upmost caution. Binary azide compounds can take on several different structures including discrete compounds, or one- two, and three-dimensional nets, leading some to dub them as "polyazides". Reactivity studies of azide compounds are relatively limited due to how sensitive they can be. The sensitivity of these compounds tends to be correlated with the amount of ionic or covalent character the azide-element bond has, with ionic character being far more stable than covalent character. Therefore, compounds such as silver or sodium azide – which have strong ionic character – tend to possess more synthetic utility than their covalent counterparts. A few other notable exceptions include polymeric networks which possess unique magnetic properties, group 13 azides which unlike most other azides decompose to nitride compounds (important materials for semiconductors), other limited uses as synthetic reagents for the transfer of azide groups, or for research into high-energy-density matter.