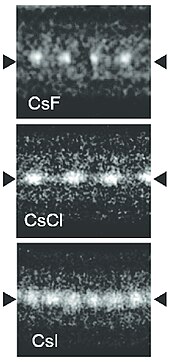
CsI crystal | |
 Scintillating CsI crystal | |
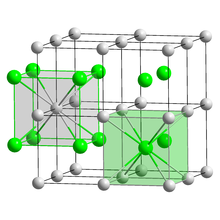 Crystal structure | |
 | |
| Names | |
|---|---|
| IUPAC name Caesium iodide | |
| Other names Cesium iodide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.223 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CsI | |
| Molar mass | 259.809 g/mol [2] |
| Appearance | white crystalline solid |
| Density | 4.51 g/cm3 [2] |
| Melting point | 632 °C (1,170 °F; 905 K) [2] |
| Boiling point | 1,280 °C (2,340 °F; 1,550 K) [2] |
| 848 g/L (25 °C) [2] | |
| −82.6·10−6 cm3/mol [3] | |
Refractive index (nD) | 1.9790 (0.3 µm) 1.7873 (0.59 µm) 1.7694 (0.75 µm) 1.7576 (1 µm) 1.7428 (5 µm) 1.7280 (20 µm) [4] |
| Structure | |
| CsCl, cP2 | |
| Pm3m, No. 221 [5] | |
a = 0.4503 nm | |
Lattice volume (V) | 0.0913 nm3 |
Formula units (Z) | 1 |
| Cubic (Cs+) Cubic (I−) | |
| Thermochemistry | |
Heat capacity (C) | 52.8 J/mol·K [6] |
Std molar entropy (S⦵298) | 123.1 J/mol·K [6] |
Std enthalpy of formation (ΔfH⦵298) | −346.6 kJ/mol [6] |
Gibbs free energy (ΔfG⦵) | −340.6 kJ/mol [6] |
| Hazards | |
| GHS labelling: | |
   | |
| Warning | |
| H315, H317, H319, H335 | |
| P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 2386 mg/kg (oral, rat) [1] |
| Related compounds | |
Other anions | Caesium fluoride Caesium chloride Caesium bromide Caesium astatide |
Other cations | Lithium iodide Sodium iodide Potassium iodide Rubidium iodide Francium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths. [7]