
Hemolysis or haemolysis, also known by several other names, is the rupturing (lysis) of red blood cells (erythrocytes) and the release of their contents (cytoplasm) into surrounding fluid. Hemolysis may occur in vivo or in vitro.

Thrombotic thrombocytopenic purpura (TTP) is a blood disorder that results in blood clots forming in small blood vessels throughout the body. This results in a low platelet count, low red blood cells due to their breakdown, and often kidney, heart, and brain dysfunction. Symptoms may include large bruises, fever, weakness, shortness of breath, confusion, and headache. Repeated episodes may occur.
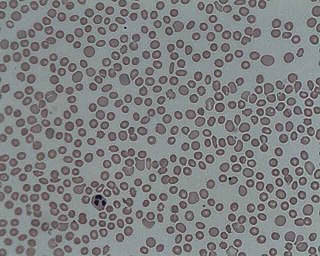
Microangiopathic hemolytic anemia (MAHA) is a microangiopathic subgroup of hemolytic anemia caused by factors in the small blood vessels. It is identified by the finding of anemia and schistocytes on microscopy of the blood film.
Thrombocytopenia is a condition characterized by abnormally low levels of platelets, also known as thrombocytes, in the blood. It is the most common coagulation disorder among intensive care patients and is seen in 20% of medical patients and a third of surgical patients.

Hemolytic–uremic syndrome (HUS) is a group of blood disorders characterized by low red blood cells, acute kidney failure, and low platelets. Initial symptoms typically include bloody diarrhea, fever, vomiting, and weakness. Kidney problems and low platelets then occur as the diarrhea progresses. Children are more commonly affected, but most children recover without permanent damage to their health, although some children may have serious and sometimes life-threatening complications. Adults, specially the elderly, may present a more complicated presentation. Complications may include neurological problems and heart failure.

von Willebrand factor (VWF) is a blood glycoprotein involved in hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, metabolic, and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis.

Hemoglobinuria is a condition in which the oxygen transport protein hemoglobin is found in abnormally high concentrations in the urine. The condition is caused by excessive intravascular hemolysis, in which large numbers of red blood cells (RBCs) are destroyed, thereby releasing free hemoglobin into the plasma. Excess hemoglobin is filtered by the kidneys, which excrete it into the urine, giving urine a purple color. Hemoglobinuria can lead to acute tubular necrosis which is an uncommon cause of a death of uni-traumatic patients recovering in the ICU.
HELLP syndrome is a complication of pregnancy; the acronym stands for Hemolysis, Elevated Liver enzymes, and Low Platelet count. It usually begins during the last three months of pregnancy or shortly after childbirth. Symptoms may include feeling tired, retaining fluid, headache, nausea, upper right abdominal pain, blurry vision, nosebleeds, and seizures. Complications may include disseminated intravascular coagulation, placental abruption, and kidney failure.

A schistocyte or schizocyte is a fragmented part of a red blood cell. Schistocytes are typically irregularly shaped, jagged, and have two pointed ends.

Nephritic syndrome is a syndrome comprising signs of nephritis, which is kidney disease involving inflammation. It often occurs in the glomerulus, where it is called glomerulonephritis. Glomerulonephritis is characterized by inflammation and thinning of the glomerular basement membrane and the occurrence of small pores in the podocytes of the glomerulus. These pores become large enough to permit both proteins and red blood cells to pass into the urine. By contrast, nephrotic syndrome is characterized by proteinuria and a constellation of other symptoms that specifically do not include hematuria. Nephritic syndrome, like nephrotic syndrome, may involve low level of albumin in the blood due to the protein albumin moving from the blood to the urine.

ADAMTS13 —also known as von Willebrand factor-cleaving protease (VWFCP)—is a zinc-containing metalloprotease enzyme that cleaves von Willebrand factor (vWf), a large protein involved in blood clotting. It is secreted into the blood and degrades large vWf multimers, decreasing their activity.
Hematologic diseases are disorders which primarily affect the blood & blood-forming organs. Hematologic diseases include rare genetic disorders, anemia, HIV, sickle cell disease & complications from chemotherapy or transfusions.
The term cryosupernatant refers to plasma from which the cryoprecipitate has been removed. It is used to treat thrombocytopenic purpura.
Shigatoxigenic Escherichia coli (STEC) and verotoxigenic E. coli (VTEC) are strains of the bacterium Escherichia coli that produce either Shiga toxin or Shiga-like toxin (verotoxin). Only a minority of the strains cause illness in humans. The ones that do are collectively known as enterohemorrhagic E. coli (EHEC) and are major causes of foodborne illness. When infecting humans, they often cause gastroenteritis, enterocolitis, and bloody diarrhea and sometimes cause a severe complication called hemolytic-uremic syndrome (HUS). The group and its subgroups are known by various names. They are distinguished from other strains of intestinal pathogenic E. coli including enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC).
Atypical hemolytic uremic syndrome (aHUS) is an extremely rare, life-threatening, progressive disease that frequently has a genetic component. In most cases it can be effectively controlled by interruption of the complement cascade. Particular monoclonal antibodies, discussed later in the article, have proven efficacy in many cases.
Onconephrology is a specialty in nephrology that deals with the study of kidney diseases in cancer patients. A nephrologist who takes care of patients with cancer and kidney disease is called an onconephrologist. This branch of nephrology encompasses nephrotoxicity associated with existing and novel chemotherapeutics, kidney disease as it pertains to stem cell transplant, paraneoplastic kidney disorders, paraproteinemias, electrolyte disorders associated with cancer, and more as discussed below.

Upshaw–Schulman syndrome (USS) is the recessively inherited form of thrombotic thrombocytopenic purpura (TTP), a rare and complex blood coagulation disease. USS is caused by the absence of the ADAMTS13 protease resulting in the persistence of ultra large von Willebrand factor multimers (ULVWF), causing episodes of acute thrombotic microangiopathy with disseminated multiple small vessel obstructions. These obstructions deprive downstream tissues from blood and oxygen, which can result in tissue damage and death. The presentation of an acute USS episode is variable but usually associated with thrombocytopenia, microangiopathic hemolytic anemia (MAHA) with schistocytes on the peripheral blood smear, fever and signs of ischemic organ damage in the brain, kidney and heart.
Ravulizumab, sold under the brand name Ultomiris, is a humanized monoclonal antibody complement inhibitor medication designed for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome. It is designed to bind to and prevent the activation of Complement component 5 (C5).
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder characterized by thrombocytopenia and microangiopathic hemolytic anemia accompanied by variable neurological dysfunction, kidney failure, and fever. It is caused by severely reduced activity of the von Willebrand factor-cleaving protease ADAMTS13. Hereditary TTP, caused by ADAMTS13 gene mutations, is much less common.









