The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.
Hepatitis D is a type of viral hepatitis caused by the hepatitis delta virus (HDV). HDV is one of five known hepatitis viruses: A, B, C, D, and E. HDV is considered to be a satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection).

Simian immunodeficiency virus (SIV) is a species of retrovirus that cause persistent infections in at least 45 species of non-human primates. Based on analysis of strains found in four species of monkeys from Bioko Island, which was isolated from the mainland by rising sea levels about 11,000 years ago, it has been concluded that SIV has been present in monkeys and apes for at least 32,000 years, and probably much longer.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.
GB virus C (GBV-C), formerly known as hepatitis G virus (HGV) and also known as human pegivirus – HPgV is a virus in the family Flaviviridae and a member of the Pegivirus, is known to infect humans, but is not known to cause human disease. Reportedly, HIV patients coinfected with GBV-C can survive longer than those without GBV-C, but the patients may be different in other ways. Research is active into the virus' effects on the immune system in patients coinfected with GBV-C and HIV.
Simian foamy virus (SFV) is a species of the genus Spumavirus that belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including prosimians, New World and Old World monkeys, as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques, and chimpanzees. As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat-hunting practices.

Murine coronavirus (M-CoV) is a virus in the genus Betacoronavirus that infects mice. Belonging to the subgenus Embecovirus, murine coronavirus strains are enterotropic or polytropic. Enterotropic strains include mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM, whereas polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis. Murine coronavirus is an important pathogen in the laboratory mouse and the laboratory rat. It is the most studied coronavirus in animals other than humans, and has been used as an animal disease model for many virological and clinical studies.

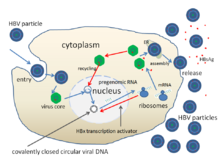
Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.

A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity. Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898, more than 11,000 of the millions of virus species have been described in detail. The study of viruses is known as virology, a subspeciality of microbiology.

Orthohepadnavirus is a genus of viruses, in the family Hepadnaviridae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: hepatitis, hepatocellular carcinoma, and cirrhosis.

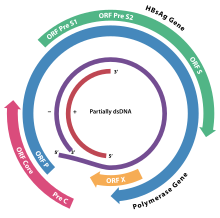
Hepatitis B virus (HBV) is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
cccDNA is a special DNA structure that arises during the propagation of some viruses in the cell nucleus and may remain permanently there. It is a double-stranded DNA that originates in a linear form that is ligated by means of DNA ligase to a covalently closed ring. In most cases, transcription of viral DNA can occur from the circular form only. The cccDNA of viruses is also known as episomal DNA or occasionally as a minichromosome.
Vesivirus is a genus of viruses, in the family Caliciviridae. Swine, sea mammals, and felines serve as natural hosts. There are two species in this genus. Diseases associated with this genus include: respiratory disease, Feline calicivirus (FCV); conjunctivitis, and respiratory disease.

A precore mutant is a variety of hepatitis B virus that does not produce hepatitis B virus e antigen (HBeAg). These mutants are important because infections caused by these viruses are difficult to treat, and can cause infections of prolonged duration and with a higher risk of liver cirrhosis. The mutations are changes in DNA bases from guanine to adenine at base position 1896 (G1896A), and from cytosine to thymine at position 1858 (C1858T) in the precore region of the viral genome.
Aichivirus A formerly Aichi virus (AiV) belongs to the genus Kobuvirus in the family Picornaviridae. Six species are apart of the genus Kobuvirus, Aichivirus A-F. Within Aichivirus A, there are six different types including human Aichi virus, canine kobuvirus, murine kobuvirus, Kathmandu sewage kobuvirus, roller kobuvirus, and feline kobuvirus. Three different genotypes are found in human Aichi virus, represented as genotype A, B, and C.
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.

Positive-strand RNA viruses are a group of related viruses that have positive-sense, single-stranded genomes made of ribonucleic acid. The positive-sense genome can act as messenger RNA (mRNA) and can be directly translated into viral proteins by the host cell's ribosomes. Positive-strand RNA viruses encode an RNA-dependent RNA polymerase (RdRp) which is used during replication of the genome to synthesize a negative-sense antigenome that is then used as a template to create a new positive-sense viral genome.

Ground squirrel hepatitis virus, abbreviated GSHV, is a partially double-stranded DNA virus that is closely related to human Hepatitis B virus (HBV) and Woodchuck hepatitis virus (WHV). It is a member of the family of viruses Hepadnaviridae and the genus Orthohepadnavirus. Like the other members of its family, GSHV has high degree of species and tissue specificity. It was discovered in Beechey ground squirrels, Spermophilus beecheyi, but also infects Arctic ground squirrels, Spermophilus parryi. Commonalities between GSHV and HBV include morphology, DNA polymerase activity in genome repair, cross-reacting viral antigens, and the resulting persistent infection with viral antigen in the blood (antigenemia). As a result, GSHV is used as an experimental model for HBV.

The woolly monkey hepatitis B virus (WMHBV) is a viral species of the Orthohepadnavirus genus of the Hepadnaviridae family. Its natural host is the woolly monkey (Lagothrix), an inhabitant of South America categorized as a New World primate. WMHBV, like other hepatitis viruses, infects the hepatocytes, or liver cells, of its host organism. It can cause hepatitis, liver necrosis, cirrhosis, and hepatocellular carcinoma. Because nearly all species of Lagothrix are threatened or endangered, researching and developing a vaccine and/or treatment for WMHBV is important for the protection of the whole woolly monkey genus.