
Treponema pallidum, formerly known as Spirochaeta pallida, is a spirochaete bacterium with various subspecies that cause the diseases syphilis, bejel, and yaws. It is transmitted only among humans. It is a helically coiled microorganism usually 6–15 μm long and 0.1–0.2 μm wide. T. pallidum's lack of either a tricarboxylic acid cycle or oxidative phosphorylation results in minimal metabolic activity. The treponemes have a cytoplasmic and an outer membrane. Using light microscopy, treponemes are visible only by using dark-field illumination. T. pallidum consists of three subspecies, T. p. pallidum, T. p. endemicum, and T. p. pertenue, each of which has a distinct associated disease.
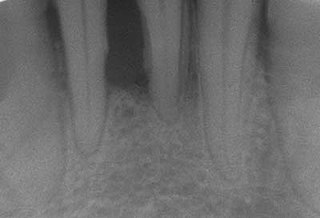
Periodontal disease, also known as gum disease, is a set of inflammatory conditions affecting the tissues surrounding the teeth. In its early stage, called gingivitis, the gums become swollen and red and may bleed. It is considered the main cause of tooth loss for adults worldwide. In its more serious form, called periodontitis, the gums can pull away from the tooth, bone can be lost, and the teeth may loosen or fall out. Bad breath may also occur.

In dentistry, calculus or tartar is a form of hardened dental plaque. It is caused by precipitation of minerals from saliva and gingival crevicular fluid (GCF) in plaque on the teeth. This process of precipitation kills the bacterial cells within dental plaque, but the rough and hardened surface that is formed provides an ideal surface for further plaque formation. This leads to calculus buildup, which compromises the health of the gingiva (gums). Calculus can form both along the gumline, where it is referred to as supragingival, and within the narrow sulcus that exists between the teeth and the gingiva, where it is referred to as subgingival.
Periodontology or periodontics is the specialty of dentistry that studies supporting structures of teeth, as well as diseases and conditions that affect them. The supporting tissues are known as the periodontium, which includes the gingiva (gums), alveolar bone, cementum, and the periodontal ligament. A periodontist is a dentist that specializes in the prevention, diagnosis and treatment of periodontal disease and in the placement of dental implants.
Dental plaque is a biofilm of microorganisms that grows on surfaces within the mouth. It is a sticky colorless deposit at first, but when it forms tartar, it is often brown or pale yellow. It is commonly found between the teeth, on the front of teeth, behind teeth, on chewing surfaces, along the gumline (supragingival), or below the gumline cervical margins (subgingival). Dental plaque is also known as microbial plaque, oral biofilm, dental biofilm, dental plaque biofilm or bacterial plaque biofilm. Bacterial plaque is one of the major causes for dental decay and gum disease.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract.
Fusobacterium nucleatum is a Gram-negative, anaerobic oral bacterium, commensal to the human oral cavity, that plays a role in periodontal disease. This organism is commonly recovered from different monocultured microbial and mixed infections in humans and animals. In health and disease, it is a key component of periodontal plaque due to its abundance and its ability to coaggregate with other bacteria species in the oral cavity.
Porphyromonas gingivalis belongs to the phylum Bacteroidota and is a nonmotile, Gram-negative, rod-shaped, anaerobic, pathogenic bacterium. It forms black colonies on blood agar.
Aggregatibacter actinomycetemcomitans is a Gram-negative, facultative anaerobe, nonmotile bacterium that is often found in association with localized aggressive periodontitis, a severe infection of the periodontium. It is also suspected to be involved in chronic periodontitis. Less frequently, A. actinomycetemcomitans is associated with nonoral infections such as endocarditis. Its role in aggressive periodontitis was first discovered by Danish-born periodontist Jørgen Slots, a professor of dentistry and microbiology at the University of Southern California School of Dentistry.

Scaling and root planing, also known as conventional periodontal therapy, non-surgical periodontal therapy or deep cleaning, is a procedure involving removal of dental plaque and calculus and then smoothing, or planing, of the (exposed) surfaces of the roots, removing cementum or dentine that is impregnated with calculus, toxins, or microorganisms, the agents that cause inflammation. It is a part of non-surgical periodontal therapy. This helps to establish a periodontium that is in remission of periodontal disease. Periodontal scalers and periodontal curettes are some of the tools involved.
Oral microbiology is the study of the microorganisms (microbiota) of the oral cavity and their interactions between oral microorganisms or with the host. The environment present in the human mouth is suited to the growth of characteristic microorganisms found there. It provides a source of water and nutrients, as well as a moderate temperature. Resident microbes of the mouth adhere to the teeth and gums to resist mechanical flushing from the mouth to stomach where acid-sensitive microbes are destroyed by hydrochloric acid.
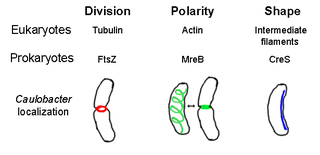
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but advances in visualization technology and structure determination led to the discovery of filaments in these cells in the early 1990s. Not only have analogues for all major cytoskeletal proteins in eukaryotes been found in prokaryotes, cytoskeletal proteins with no known eukaryotic homologues have also been discovered. Cytoskeletal elements play essential roles in cell division, protection, shape determination, and polarity determination in various prokaryotes.

Gingivitis is a non-destructive disease that causes inflammation of the gums. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms that is attached to tooth surfaces, termed plaque-induced gingivitis. Most forms of gingivitis are plaque-induced.

Porphyromonas is a Gram-negative, non-spore-forming, obligately anaerobic and non-motile genus from the family Porphyromonadaceae. There were 16 different Porphyromonas species documented as of 2015, which reside in both animal and human reservoirs. It was discovered more recently that Porphyromonas also exist in the environment, albeit to a lesser extent. This genus is notably implicated in the modulation of oral cavity, respiratory tract, and gastrointestinal tract disease states. It is suggested that Porphyromonas either operate as benign bacteria pertinent to host immunity or are potential pathobionts that opportunistically provoke diseased states when homeostasis is disrupted. Despite its characterization not being fully elucidated due to sparse research, various studies report the prevalence of this genus at 58.7% in healthy states compared with 41.3% in diseased states.
Tannerella forsythia is an anaerobic, Gram-negative bacterial species of the Bacteroidota phylum. It has been implicated in periodontal diseases and is a member of the red complex of periodontal pathogens. T. forsythia was previously named Bacteroides forsythus and Tannerella forsythensis.
Chronic periodontitis is one of the seven categories of periodontitis as defined by the American Academy of Periodontology 1999 classification system. Chronic periodontitis is a common disease of the oral cavity consisting of chronic inflammation of the periodontal tissues that is caused by the accumulation of profuse amounts of dental plaque. Periodontitis initially begins as gingivitis and can progress onto chronic and subsequent aggressive periodontitis according to the 1999 classification.
Aggressive periodontitis describes a type of periodontal disease and includes two of the seven classifications of periodontitis as defined by the 1999 classification system:
- Localized aggressive periodontitis (LAP)
- Generalized aggressive periodontitis (GAP)
Well studied Periodontal pathogens are bacteria that have been shown to significantly contribute to periodontitis.
Treponema socranskii was isolated from gum swabs of people with periodontitis and clinically-induced periodontitis. It is a motile, helically coiled, obligate anaerobe that grows best at 37 °C, and is a novel member of its genus because of its ability to ferment molecules that other Treponema species cannot. T. socranskii’s growth is positively correlated with gingival inflammation, which indicates that it is a leading cause of gingivitis and periodontitis.







