Aromatic compounds are those chemical compounds that contain one or more rings with pi electrons delocalized all the way around them. In contrast to compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing solely carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when part of a larger compound.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl groups are chemically aromatic and have equal bond lengths between carbon atoms in the ring.

Creosote is a category of carbonaceous chemicals formed by the distillation of various tars and pyrolysis of plant-derived material, such as wood or fossil fuel. They are typically used as preservatives or antiseptics.
Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds, which are categorized as phenols. Depending on the temperature, cresols can be solid or liquid because they have melting points not far from room temperature. Like other types of phenols, they are slowly oxidized by long exposure to air, and the impurities often give samples of cresols a yellowish to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name cresol reflects their structure, being phenols, and their traditional source, creosote.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.
Dihydroxybenzenes are organic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three isomer: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.
There are three isomers of toluidine, which are organic compounds. These isomers are o-toluidine, m-toluidine, and p-toluidine, with the prefixed letter abbreviating, respectively, ortho; meta; and para. All three are aryl amines whose chemical structures are similar to aniline except that a methyl group is substituted onto the benzene ring. The difference between these three isomers is the position where the methyl group (–CH3) is bonded to the ring relative to the amino functional group (–NH2); see illustration of the chemical structures below.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
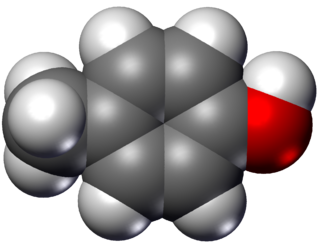
para-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of o-cresol and m-cresol.

meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups and a hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.

Amylmetacresol (AMC) is an antiseptic used to treat infections of the mouth and throat. It is used as an active pharmaceutical ingredient in Strepsils, Cēpacol, Gorpils and Lorsept throat lozenges.
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
The molecular formula C7H8O (molar mass: 108.13 g/mol, exact mass: 108.057515 u) may refer to:

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch. In this organic reduction of aromatic rings in liquid ammonia with sodium, lithium, or potassium and an alcohol, such as ethanol and tert-butanol. This reaction is unlike catalytic hydrogenation, which usually reduces the aromatic ring all the way to a cyclohexane.
ortho-Cresol, also 2-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.

Dinitro-ortho-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. DNOC and some related derivatives have been used as herbicides.
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, and alkylation and acylation Friedel–Crafts reaction.








