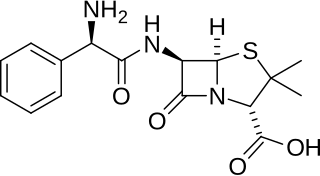
Ampicillin is an antibiotic belonging to the aminopenicillin class of the penicillin family. The drug is used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously.

Macrolides are a class of mostly natural products with a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic.

Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.

Clindamycin is an antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media, and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant Staphylococcus aureus (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.
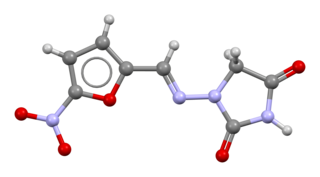
Nitrofurantoin is an antibacterial medication used to treat urinary tract infections, but it is not as effective for kidney infections. It is taken by mouth.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.
Mycoplasma pneumonia is a form of bacterial pneumonia caused by the bacterium Mycoplasma pneumoniae.

Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics. In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.

Cefadroxil is a broad-spectrum antibiotic of the cephalosporin type, effective in Gram-positive and Gram-negative bacterial infections. It is a bactericidal antibiotic.

Lincomycin is a lincosamide antibiotic that comes from the actinomycete Streptomyces lincolnensis. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964.

Marbofloxacin is a carboxylic acid derivative third generation fluoroquinolone antibiotic. It is used in veterinary medicine under the brand names Marbocyl, Forcyl, Marbo vet and Zeniquin. A formulation of marbofloxacin combined with clotrimazole and dexamethasone is available under the name Aurizon.

Cefotiam is a parenteral third-generation cephalosporin antibiotic. It has broad-spectrum activity against Gram-positive and Gram-negative bacteria. As a beta-lactam, its bactericidal activity results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins.

Cefquinome is a fourth-generation cephalosporin with pharmacological and antibacterial properties valuable in the treatment of coliform mastitis and other infections. It is only used in veterinary applications.

Enrofloxacin, sold under the brand name Baytril, among others, is a fluoroquinolone antibiotic used for the treatment of animals. It is a bactericidal agent.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.

Oleandomycin is a macrolide antibiotic. It is synthesized from strains of Streptomyces antibioticus. It is weaker than erythromycin.

Spiramycin is a macrolide antibiotic and antiparasitic. It is used to treat toxoplasmosis and various other infections of soft tissues. Although used in Europe, Canada and Mexico, spiramycin is still considered an experimental drug in the United States, but can sometimes be obtained by special permission from the FDA for toxoplasmosis in the first trimester of pregnancy. Another treatment option are a combination of pyrimethamine and sulfadiazine.

Bovine mastitis is the persistent, inflammatory reaction of the udder tissue due to physical trauma or microorganisms infections. Mastitis, a potentially fatal mammary gland infection, is the most common disease in dairy cattle in the United States and worldwide. It is also the most costly disease to the dairy industry. Milk from cows suffering from mastitis has an increased somatic cell count. Prevention and control of mastitis requires consistency in sanitizing the cow barn facilities, proper milking procedure and segregation of infected animals. Treatment of the disease is carried out by penicillin injection in combination with sulphar drug.
Mycoplasma bovis is one of 126 species of genus Mycoplasma. It is the smallest living cell and anaerobic organism in nature. It does not contain any cell wall and is therefore resistant to penicillin and other beta lactam antibiotics.

















