Related Research Articles

Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved venom apparatus, such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism.

Bradykinin (BK) (Greek brady-, slow; -kinin, kīn(eîn) to move) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin consists of nine amino acids, and is a physiologically and pharmacologically active peptide of the kinin group of proteins.
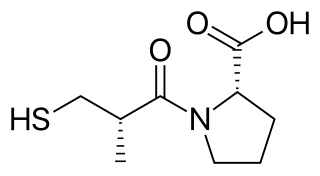
Captopril, sold under the brand name Capoten among others, is an angiotensin-converting enzyme (ACE) inhibitor used for the treatment of hypertension and some types of congestive heart failure. Captopril was the first oral ACE inhibitor found for the treatment of hypertension. It does not cause fatigue as associated with beta-blockers. Due to the adverse drug event of causing hyperkalemia, as seen with most ACE Inhibitors, the medication is usually paired with a diuretic.

Bothrops atrox — also known as the common lancehead, fer-de-lance, barba amarilla and mapepire balsain — is a highly venomous pit viper species found in the tropical lowlands of northern South America east of the Andes, as well as the Caribbean island of Trinidad. No subspecies are currently recognized.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is injected by unique fangs during a bite, whereas some species are also able to spit venom.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.
Ziconotide, sold under the brand name Prialt, also called intrathecal ziconotide (ITZ) because of its administration route, is an atypical analgesic agent for the amelioration of severe and chronic pain. Derived from Conus magus, a cone snail, it is the synthetic form of an ω-conotoxin peptide. It is 1,000 times as powerful as morphine.

A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.

The Chinese red-headed centipede, also known as the Chinese red head, is a centipede from East Asia. It averages 20 cm (8 in) in length and lives in damp environments.

Tirofiban, sold under the brand name Aggrastat, is an antiplatelet medication. It belongs to a class of antiplatelets named glycoprotein IIb/IIIa inhibitors. Tirofiban is a small molecule inhibitor of the protein-protein interaction between fibrinogen and the platelet integrin receptor GP IIb/IIIa and is the first drug candidate whose origins can be traced to a pharmacophore-based virtual screening lead.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.

Teprotide is nonapeptide which has been isolated from the snake Bothrops jararaca. It is an angiotensin converting enzyme inhibitor (ACE inhibitor) which inhibits the conversion of angiotensin I to angiotensin II and may potentiate some of the pharmacological actions of bradykinin. It has a molecular formula of C53H76N14O12 and has been investigated as an antihypertension agent.
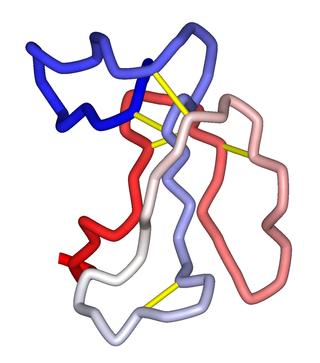
α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
Tamulotoxin is a venomous neurotoxin from the Indian Red Scorpion.

Venom in snakes and some lizards is a form of saliva that has been modified into venom over its evolutionary history. In snakes, venom has evolved to kill or subdue prey, as well as to perform other diet-related functions. While snakes occasionally use their venom in self defense, this is not believed to have had a strong effect on venom evolution. The evolution of venom is thought to be responsible for the enormous expansion of snakes across the globe.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).

Three-finger proteins or three-finger protein domains are a protein superfamily consisting of small, roughly 60-80 amino acid residue protein domains with a common tertiary structure: three beta strand loops extended from a hydrophobic core stabilized by disulfide bonds. The family is named for the outstretched "fingers" of the three loops. Members of the family have no enzymatic activity, but are capable of forming protein-protein interactions with high specificity and affinity. The founding members of the family, also the best characterized by structure, are the three-finger toxins found in snake venom, which have a variety of pharmacological effects, most typically by disruption of cholinergic signaling. The family is also represented in non-toxic proteins, which have a wide taxonomic distribution; 3FP domains occur in the extracellular domains of some cell-surface receptors as well as in GPI-anchored and secreted globular proteins, usually involved in signaling.
Venomics is the large-scale study of proteins associated with venom. Venom is a toxic substance secreted by animals, which is typically injected either offensively or defensively into prey or aggressors, respectively.
References
- ↑ Gottlieb Z (3 November 2010). "These 5 Poisons May Save Your Life". Popular Mechanics. Retrieved 2018-02-22.
- 1 2 3 4 5 6 Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG (April 2013). "Complex cocktails: the evolutionary novelty of venoms". Trends in Ecology & Evolution. 28 (4): 219–29. doi:10.1016/j.tree.2012.10.020. PMID 23219381.
- ↑ Harvey AL (December 2014). "Toxins and drug discovery". Toxicon. 92: 193–200. doi: 10.1016/j.toxicon.2014.10.020 . PMID 25448391.
- ↑ "On The Origin of Venom". Phenomena. 2013-01-09. Archived from the original on January 13, 2013. Retrieved 2018-04-21.
- 1 2 3 4 Utkin YN (May 2015). "Animal venom studies: Current benefits and future developments". World Journal of Biological Chemistry. 6 (2): 28–33. doi: 10.4331/wjbc.v6.i2.28 . PMC 4436903 . PMID 26009701.
- ↑ Slagboom J, Kool J, Harrison RA, Casewell NR (June 2017). "Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise". British Journal of Haematology. 177 (6): 947–959. doi:10.1111/bjh.14591. PMC 5484289 . PMID 28233897.
- 1 2 McGivern JG (February 2007). "Ziconotide: a review of its pharmacology and use in the treatment of pain". Neuropsychiatric Disease and Treatment. 3 (1): 69–85. doi: 10.2147/nedt.2007.3.1.69 . PMC 2654521 . PMID 19300539.
- ↑ "Prialt solution for infusion - Summary of Product Characteristics (SmPC) - (eMC)". Electronic Medicines Compendium. January 2017. Retrieved 21 April 2018.
- 1 2 Saab F, Ionescu C, Schweiger MJ (March 2012). "Bleeding risk and safety profile related to the use of eptifibatide: a current review". Expert Opinion on Drug Safety. 11 (2): 315–24. doi:10.1517/14740338.2012.650164. PMID 22233272. S2CID 24282097.
- ↑ Pothineni NV, Watts TE, Ding Z, Dai Y, Deshmukh AJ (2016). "Eptifibatide-Induced Thrombocytopenia--When Inhibitor Turns Killer". American Journal of Therapeutics . 23 (1): e298–9. doi:10.1097/01.mjt.0000438283.01797.1a. PMID 24368608.
- ↑ Shah I, Khan SO, Malhotra S, Fischell T (June 2010). "Eptifibatide: The evidence for its role in the management of acute coronary syndromes". Core Evidence. 4: 49–65. doi: 10.2147/ce.s6008 . PMC 2899786 . PMID 20694065.
- 1 2 Raufman, JP (16 January 1996). "Bioactive peptides from lizard venoms". Regulatory Peptides. 61 (1): 1–18. doi:10.1016/0167-0115(96)00135-8. PMID 8701022. S2CID 5293453.
- ↑ "Byetta 10 micrograms solution for injection, prefilled pen - Summary of Product Characteristics". Electronic Medicines Compendium. 30 March 2017. Retrieved 21 April 2018.
- ↑ "Bydureon 2 mg powder and solvent for prolonged-release suspension for injection in pre-filled pen - Summary of Product Characteristics". Electronic Medicines Compendium. 10 November 2017. Retrieved 21 April 2018.
- ↑ Serrano, SM (February 2013). "The long road of research on snake venom serine proteinases". Toxicon. 62: 19–26. doi:10.1016/j.toxicon.2012.09.003. PMID 23010164.