Related Research Articles

Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.
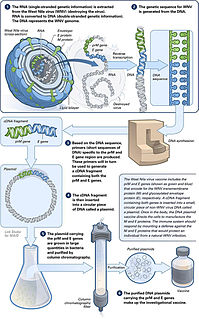
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

Cytokines are a broad and loose category of small proteins important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents. Their definite distinction from hormones is still part of ongoing research.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.
Interleukins (ILs) are a group of cytokines that were first seen to be expressed by white blood cells (leukocytes). ILs can be divided into four major groups based on distinguishing structural features. However, their amino acid sequence similarity is rather weak. The human genome encodes more than 50 interleukins and related proteins.
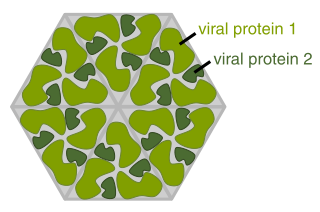
A viral protein is both a component and a product of a virus. Viral proteins are grouped according to their functions, and groups of viral proteins include structural proteins, nonstructural proteins, regulatory proteins, and accessory proteins. Viruses are non-living and do not have the means to reproduce on their own, instead depending on their host cell's resources in order to reproduce. Thus, viruses do not code for many of their own viral proteins, and instead use the host cell's machinery to produce the viral proteins they require for replication.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

The innate, or nonspecific, immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the dominant immune system response found in plants, fungi, insects, and primitive multicellular organisms.

Molluscum contagiosum virus (MCV) is a DNA poxvirus that causes the human skin infection molluscum contagiosum. Molluscum contagiosum affects about 200,000 people a year, about 1% of all diagnosed skin diseases. Diagnosis is based on the size and shape of the skin lesions and can be confirmed with a biopsy, as the virus cannot be routinely cultured. Molluscum contagiosum virus is the only species in the genus Molluscipoxvirus. MCV is a member of the subfamily Chordopoxvirinae of family Poxviridae. Other commonly known viruses that reside in the subfamily Chordopoxvirinae are variola virus and monkeypox virus.
Interleukin-15 (IL-15) is a cytokine with structural similarity to Interleukin-2 (IL-2). Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain. IL-15 is secreted by mononuclear phagocytes following infection by virus(es). This cytokine induces the proliferation of natural killer cells, i.e. cells of the innate immune system whose principal role is to kill virally infected cells.

Interleukin-26 (IL-26) is a protein that in humans is encoded by the IL26 gene.

Interleukin 24 (IL-24) is a protein in the interleukin family, a type of cytokine signaling molecule in the immune system. In humans, this protein is encoded by the IL24 gene.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.

A sole member makes up the type II interferons (IFNs) that is called IFN-γ (gamma). Mature IFN-γ is an anti-parallel homodimer, which binds to the IFN-γ receptor (IFNGR) complex to elicit a signal within its target cell. IFNGR is made up of two subunits each of molecules designated IFNGR1 and IFNGR2.
The type III interferon group is a group of anti-viral cytokines, that consists of four IFN-λ (lambda) molecules called IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4. They were discovered in 2003. Their function is similar to that of type I interferons, but is less intense and serves mostly as a first-line defense against viruses in the epithelium.
Interleukin-28 receptor is a type II cytokine receptor found largely in epithelial cells. It binds type 3 interferons, interleukin-28 A, Interleukin-28B, interleukin 29 and interferon lambda 4. It consists of an α chain and shares a common β subunit with the interleukin-10 receptor. Binding to the interleukin-28 receptor, which is restricted to select cell types, is important for fighting infection. Binding of the type 3 interferons to the receptor results in activation of the JAK/STAT signaling pathway.
Interleukin-17 receptor (IL-17R) is a cytokine receptor which belongs to new subfamily of receptors binding proinflammatory cytokine interleukin 17A, a member of IL-17 family ligands produced by T helper 17 cells (Th17). IL-17R family consists of 5 members: IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. Functional IL-17R is a transmembrane receptor complex usually consisting of one IL-17RA, which is a founding member of the family, and second other family subunit, thus forming heteromeric receptor binding different ligands. IL-17A, a founding member of IL-17 ligand family binds to heteromeric IL-17RA/RC receptor complex. IL-17RB binds preferentially IL-17B and IL-17E and heteromeric IL-17RA/RE complex binds IL-17C. However, there is still unknown ligand for IL-17RD. The first identified member IL-17RA is located on human chromosome 22, whereas other subunits IL-17RB to IL-17RD are encoded within human chromosome 3.

The Interleukin-1 family is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.
B13R is a protein expressed by vaccinia virus.
References
- 1 2 McFadden, Grant (June 2000). "Virus "Star Wars"". Science & Medicine (7): 38.
- 1 2 Alcami, A (January 2003). "Viral mimicry of cytokines, chemokines and their receptors". Nature Reviews. Immunology. 3 (1): 36–50. doi:10.1038/nri980. PMID 12511874. S2CID 23531422.
- 1 2 Lucas, A; McFadden, G (15 October 2004). "Secreted immunomodulatory viral proteins as novel biotherapeutics". Journal of Immunology. 173 (8): 4765–74. doi: 10.4049/jimmunol.173.8.4765 . PMID 15470015.
- ↑ "Dr. Bernard Moss Wins Bristol-Myers Squibb Award". National Institute of Allergy and Infectious Diseases. National Institutes of Health. 16 November 2000. Archived from the original on 20 December 2013. Retrieved 24 March 2015.
- 1 2 Kotwal, GJ; Moss, B (8 September 1988). "Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins". Nature. 335 (6186): 176–8. Bibcode:1988Natur.335..176K. doi:10.1038/335176a0. PMID 3412473. S2CID 7963129.
- ↑ Henderson, edited by Gerry A. Higgs, Brian (2000). Novel cytokine inhibitors. Basel: Birkhäuser Verlag. pp. 245–7. ISBN 9783764359423.
{{cite book}}:|first1=has generic name (help) - ↑ Odom, MR; Hendrickson, RC; Lefkowitz, EJ (September 2009). "Poxvirus protein evolution: family wide assessment of possible horizontal gene transfer events". Virus Research. 144 (1–2): 233–49. doi:10.1016/j.virusres.2009.05.006. PMC 2779260 . PMID 19464330.
- ↑ Fickenscher, H; Hör, S; Küpers, H; Knappe, A; Wittmann, S; Sticht, H (February 2002). "The interleukin-10 family of cytokines". Trends in Immunology. 23 (2): 89–96. doi:10.1016/s1471-4906(01)02149-4. PMID 11929132.