
A Carnot heat engine is a theoretical heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates.

Horsepower (hp) is a unit of measurement of power, or the rate at which work is done, usually in reference to the output of engines or motors. There are many different standards and types of horsepower. Two common definitions used today are the imperial horsepower as in "hp" or "bhp" which is about 745.7 watts, and the metric horsepower as in "cv" or "PS" which is approximately 735.5 watts.

In thermodynamics, the specific heat capacity of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat capacity or as the specific heat. More formally it is the heat capacity of a sample of the substance divided by the mass of the sample. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of 1 kg of water by 1 K is 4184 joules, so the specific heat capacity of water is 4184 J⋅kg−1⋅K−1.

Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.

The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form:

An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines.
The density of air or atmospheric density, denoted ρ, is the mass per unit volume of Earth's atmosphere. Air density, like air pressure, decreases with increasing altitude. It also changes with variations in atmospheric pressure, temperature and humidity. At 101.325 kPa (abs) and 20 °C, air has a density of approximately 1.204 kg/m3 (0.0752 lb/cu ft), according to the International Standard Atmosphere (ISA). At 101.325 kPa (abs) and 15 °C (59 °F), air has a density of approximately 1.225 kg/m3 (0.0765 lb/cu ft), which is about 1⁄800 that of water, according to the International Standard Atmosphere (ISA). Pure liquid water is 1,000 kg/m3 (62 lb/cu ft).

A compressor is a mechanical device that increases the pressure of a gas by reducing its volume. An air compressor is a specific type of gas compressor.
Exergy, often referred to as "available energy" or "useful work potential", is a fundamental concept in the field of thermodynamics and engineering. It plays a crucial role in understanding and quantifying the quality of energy within a system and its potential to perform useful work. Exergy analysis has widespread applications in various fields, including energy engineering, environmental science, and industrial processes.
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is named after Rudolf Clausius and Benoît Paul Émile Clapeyron. However, this relation was in fact originally derived by Sadi Carnot in his Reflections on the Motive Power of Fire, which was published in 1824 but largely ignored until it was rediscovered by Clausius, Clapeyron, and Lord Kelvin decades later. Kelvin said of Carnot's argument that "nothing in the whole range of Natural Philosophy is more remarkable than the establishment of general laws by such a process of reasoning."
The mean effective pressure (MEP) is a quantity relating to the operation of a reciprocating engine and is a measure of an engine's capacity to do work that is independent of engine displacement. Despite having the dimension of pressure, MEP cannot be measured. When quoted as an indicated mean effective pressure (IMEP), it may be thought of as the average pressure acting on a piston during the different portions of its cycle. When friction losses are subtracted from the IMEP, the result is the brake mean effective pressure (BMEP).
A binary cycle is a method for generating electrical power from geothermal resources and employs two separate fluid cycles, hence binary cycle. The primary cycle extracts the geothermal energy from the reservoir, and secondary cycle converts the heat into work to drive the generator and generate electricity.

A thermodynamic cycle consists of linked sequences of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function.

In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
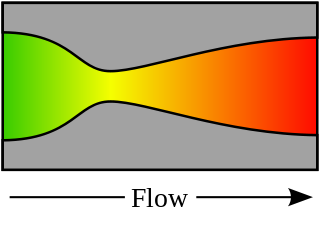
A rocket engine nozzle is a propelling nozzle used in a rocket engine to expand and accelerate combustion products to high supersonic velocities.
In aerospace engineering, concerning aircraft, rocket and spacecraft design, overall propulsion system efficiency is the efficiency with which the energy contained in a vehicle's fuel is converted into kinetic energy of the vehicle, to accelerate it, or to replace losses due to aerodynamic drag or gravity. Mathematically, it is represented as , where is the cycle efficiency and is the propulsive efficiency.
In mechanical engineering, the Beale number is a parameter that characterizes the performance of Stirling engines. It is often used to estimate the power output of a Stirling engine design. For engines operating with a high temperature differential, typical values for the Beale number are in the range 0.11−0.15; where a larger number indicates higher performance.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that transmits heat from one location at a certain temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. The operating principles in both cases are the same; energy is used to move heat from a colder place to a warmer place.
Scale of temperature is a methodology of calibrating the physical quantity temperature in metrology. Empirical scales measure temperature in relation to convenient and stable parameters or reference points, such as the freezing and boiling point of water. Absolute temperature is based on thermodynamic principles: using the lowest possible temperature as the zero point, and selecting a convenient incremental unit.

Entropy production is the amount of entropy which is produced during heat process to evaluate the efficiency of the process.









