
A heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.
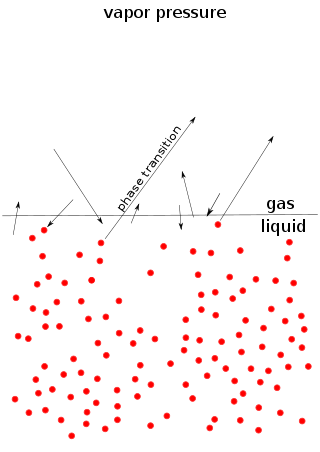
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.

A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, and hydraulic motors that are assembled into hydraulic machinery, hydraulic drive systems, etc. In pneumatics, the working fluid is air or another gas which transfers force between pneumatic components such as compressors, vacuum pumps, pneumatic cylinders, and pneumatic motors. In pneumatic systems, the working gas also stores energy because it is compressible.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, absorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.
A binary cycle is a method for generating electrical power from geothermal resources and employs two separate fluid cycles, hence binary cycle. The primary cycle extracts the geothermal energy from the reservoir, and secondary cycle converts the heat into work to drive the generator and generate electricity.

A thermodynamic cycle consists of linked sequences of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) uses are discussed in this article. In simple terms, an economizer is a heat exchanger.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

A turboexpander, also referred to as a turbo-expander or an expansion turbine, is a centrifugal or axial-flow turbine, through which a high-pressure gas is expanded to produce work that is often used to drive a compressor or generator.

A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.

A transcritical cycle is a closed thermodynamic cycle where the working fluid goes through both subcritical and supercritical states. In particular, for power cycles the working fluid is kept in the liquid region during the compression phase and in vapour and/or supercritical conditions during the expansion phase. The ultrasupercritical steam Rankine cycle represents a widespread transcritical cycle in the electricity generation field from fossil fuels, where water is used as working fluid. Other typical applications of transcritical cycles to the purpose of power generation are represented by organic Rankine cycles, which are especially suitable to exploit low temperature heat sources, such as geothermal energy, heat recovery applications or waste to energy plants. With respect to subcritical cycles, the transcritical cycle exploits by definition higher pressure ratios, a feature that ultimately yields higher efficiencies for the majority of the working fluids. Considering then also supercritical cycles as a valid alternative to the transcritical ones, the latter cycles are capable of achieving higher specific works due to the limited relative importance of the work of compression work. This evidences the extreme potential of transcritical cycles to the purpose of producing the most power with the least expenditure.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that allows for the transmission of heat from one location at a lower temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. In either case, the operating principles are similar. Heat is moved from a cold place to a warm place.

In thermal engineering, the organic Rankine cycle (ORC) is a type of thermodynamic cycle. It is a variation of the Rankine cycle named for its use of an organic, high-molecular-mass fluid whose vaporization temperature is lower than that of water. The fluid allows heat recovery from lower-temperature sources such as biomass combustion, industrial waste heat, geothermal heat, solar ponds etc. The low-temperature heat is converted into useful work, that can itself be converted into electricity.
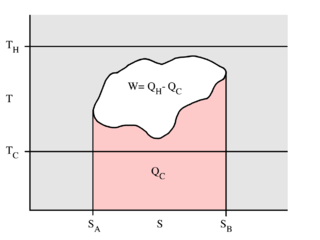
In thermodynamics, a temperature–entropy (T–s) diagram is a thermodynamic diagram used to visualize changes to temperature and specific entropy during a thermodynamic process or cycle as the graph of a curve. It is a useful and common tool, particularly because it helps to visualize the heat transfer during a process. For reversible (ideal) processes, the area under the T–s curve of a process is the heat transferred to the system during that process.
In refrigeration, flash-gas is refrigerant in gas form produced spontaneously when the condensed liquid is subjected to boiling. The presence of flash-gas in the liquid lines reduces the efficiency of the refrigeration cycle. It can also lead several expansion systems to work improperly, and increase superheating at the evaporator. This is normally perceived as an unwanted condition caused by dissociation between the volume of the system, and the pressures and temperatures that allow the refrigerant to be liquid. Flash-gas must not be confused with lack of condensation, but special gear such as receivers, internal heat exchangers, insulation, and refrigeration cycle optimizers may improve condensation and avoid gas in the liquid lines.

The Hygroscopic cycle is a thermodynamic cycle converting thermal energy into mechanical power by the means of a steam turbine. It is similar to the Rankine cycle using water as the motive fluid but with the novelty of introducing salts and their hygroscopic properties for the condensation. The salts are desorbed in the boiler or steam generator, where clean steam is released and superheated in order to be expanded and generate power through the steam turbine. Boiler blowdown with the concentrated hygroscopic compounds is used thermally to pre-heat the steam turbine condensate, and as reflux in the steam-absorber.

Non ideal compressible fluid dynamics (NICFD), or non ideal gas dynamics, is a branch of fluid mechanics studying the dynamic behavior of fluids not obeying ideal-gas thermodynamics. It is for example the case of dense vapors, supercritical flows and compressible two-phase flows. With the term dense vapors, we indicate all fluids in the gaseous state characterized by thermodynamic conditions close to saturation and the critical point. Supercritical fluids feature instead values of pressure and temperature larger than their critical values, whereas two-phase flows are characterized by the simultaneous presence of both liquid and gas phases.













