Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses include treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants and milkweed plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment.

A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.
Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.

Phospholamban, also known as PLN or PLB, is a micropeptide protein that in humans is encoded by the PLN gene. Phospholamban is a 52-amino acid integral membrane protein that regulates the calcium (Ca2+) pump in cardiac muscle cells.
Myocardial contractility represents the innate ability of the heart muscle (cardiac muscle or myocardium) to contract. It is the maximum attainable value for the force of contraction of a given heart. The ability to produce changes in force during contraction result from incremental degrees of binding between different types of tissue, that is, between filaments of myosin (thick) and actin (thin) tissue. The degree of binding depends upon the concentration of calcium ions in the cell. Within an in vivo intact heart, the action/response of the sympathetic nervous system is driven by precisely timed releases of a catecholamine, which is a process that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. The factors causing an increase in contractility work by causing an increase in intracellular calcium ions (Ca++) during contraction.
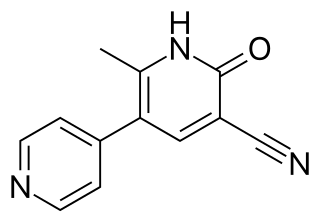
Milrinone, sold under the brand name Primacor, is a pulmonary vasodilator used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.
Levosimendan (INN) is a calcium sensitizer used in the management of acutely decompensated congestive heart failure. It is marketed under the trade name Simdax. Overall the drug has a two fold mechanism of action. It leads to greater inotrophy by increasing the calcium sensitivity as it binds to troponin and this results in a greater positive inotrophic force. Secondly, the drug is able to open ATP sensitive potassium channels in vascular smooth muscle cells, and the vascular dilatory effects of the drug lead to a decreased preload and afterload, putting less work on the heart. This drug is in the process of review by the FDA but has not been approved for use in the United States yet.

The PDE2 enzyme is one of 21 different phosphodiesterases (PDE) found in mammals. These different PDEs can be subdivided to 11 families. The different PDEs of the same family are functionally related despite the fact that their amino acid sequences show considerable divergence. The PDEs have different substrate specificities. Some are cAMP selective hydrolases, others are cGMP selective hydrolases and the rest can hydrolyse both cAMP and cGMP.

k-Strophanthidin is a cardenolide found in species of the genus Strophanthus. It is the aglycone of k-strophanthin, an analogue of ouabain. k-strophanthin is found in the ripe seeds of Strophanthus kombé and in the lily Convallaria.

A PDE3 inhibitor is a drug which inhibits the action of the phosphodiesterase enzyme PDE3. They are used for the therapy of acute heart failure and cardiogenic shock.

Pimobendan, sold under the brand name Vetmedin among others, is a veterinary medication. It is a calcium sensitizer and a selective inhibitor of phosphodiesterase 3 (PDE3) with positive inotropic and vasodilator effects.
Management of heart failure requires a multimodal approach. It involves a combination of lifestyle modifications, medications, and possibly the use of devices or surgery.

Quazinone (Dozonone) is a cardiotonic and vasodilator drug which was developed and marketed in the 1980s for the treatment of heart disease. It acts as a selective PDE3 inhibitor. It is no longer available.

Omecamtiv mecarbil (INN), previously referred to as CK-1827452, is a cardiac-specific myosin activator. It is an experimental drug being studied for a potential role in the treatment of left ventricular systolic heart failure.

Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
Arnold Martin Katz was an American medical doctor, professor of cardiology, medical researcher, and author of medical textbooks and research articles.