Related Research Articles

In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural, or evolutionary relationships between the sequences. Aligned sequences of nucleotide or amino acid residues are typically represented as rows within a matrix. Gaps are inserted between the residues so that identical or similar characters are aligned in successive columns. Sequence alignments are also used for non-biological sequences, such as calculating the distance cost between strings in a natural language or in financial data.
In bioinformatics, sequence analysis is the process of subjecting a DNA, RNA or peptide sequence to any of a wide range of analytical methods to understand its features, function, structure, or evolution. Methodologies used include sequence alignment, searches against biological databases, and others.
In computational biology, gene prediction or gene finding refers to the process of identifying the regions of genomic DNA that encode genes. This includes protein-coding genes as well as RNA genes, but may also include prediction of other functional elements such as regulatory regions. Gene finding is one of the first and most important steps in understanding the genome of a species once it has been sequenced.
The Bioinformatics Centre is an interdisciplinary research in bioinformatics at the University of Copenhagen (UCPH). The centre is housed in the Section for Computational and RNA Biology at the Department of Biology within the Faculty of Science.
In academia, computational immunology is a field of science that encompasses high-throughput genomic and bioinformatics approaches to immunology. The field's main aim is to convert immunological data into computational problems, solve these problems using mathematical and computational approaches and then convert these results into immunologically meaningful interpretations.
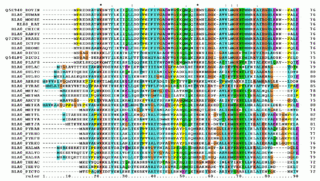
Multiple sequence alignment (MSA) may refer to the process or the result of sequence alignment of three or more biological sequences, generally protein, DNA, or RNA. In many cases, the input set of query sequences are assumed to have an evolutionary relationship by which they share a linkage and are descended from a common ancestor. From the resulting MSA, sequence homology can be inferred and phylogenetic analysis can be conducted to assess the sequences' shared evolutionary origins. Visual depictions of the alignment as in the image at right illustrate mutation events such as point mutations that appear as differing characters in a single alignment column, and insertion or deletion mutations that appear as hyphens in one or more of the sequences in the alignment. Multiple sequence alignment is often used to assess sequence conservation of protein domains, tertiary and secondary structures, and even individual amino acids or nucleotides.
Nucleic acid structure prediction is a computational method to determine secondary and tertiary nucleic acid structure from its sequence. Secondary structure can be predicted from one or several nucleic acid sequences. Tertiary structure can be predicted from the sequence, or by comparative modeling.
Rfam is a database containing information about non-coding RNA (ncRNA) families and other structured RNA elements. It is an annotated, open access database originally developed at the Wellcome Trust Sanger Institute in collaboration with Janelia Farm, and currently hosted at the European Bioinformatics Institute. Rfam is designed to be similar to the Pfam database for annotating protein families.

HMMER is a free and commonly used software package for sequence analysis written by Sean Eddy. Its general usage is to identify homologous protein or nucleotide sequences, and to perform sequence alignments. It detects homology by comparing a profile-HMM to either a single sequence or a database of sequences. Sequences that score significantly better to the profile-HMM compared to a null model are considered to be homologous to the sequences that were used to construct the profile-HMM. Profile-HMMs are constructed from a multiple sequence alignment in the HMMER package using the hmmbuild program. The profile-HMM implementation used in the HMMER software was based on the work of Krogh and colleagues. HMMER is a console utility ported to every major operating system, including different versions of Linux, Windows, and macOS.

Richard Michael Durbin is a British computational biologist and Al-Kindi Professor of Genetics at the University of Cambridge. He also serves as an associate faculty member at the Wellcome Sanger Institute where he was previously a senior group leader.

Sean Roberts Eddy is Professor of Molecular & Cellular Biology and of Applied Mathematics at Harvard University. Previously he was based at the Janelia Research Campus from 2006 to 2015 in Virginia. His research interests are in bioinformatics, computational biology and biological sequence analysis. As of 2016 projects include the use of Hidden Markov models in HMMER, Infernal Pfam and Rfam.

Cyrus Homi Chothia was an English biochemist who was an emeritus scientist at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) at the University of Cambridge and emeritus fellow of Wolfson College, Cambridge.

Alfonso Valencia is a Spanish biologist, ICREA Professor, current director of the Life Sciences department at Barcelona Supercomputing Center. and of Spanish National Bioinformatics Institute (INB-ISCIII). From 2015-2018, he was President of the International Society for Computational Biology. His research is focused on the study of biomedical systems with computational biology and bioinformatics approaches.

Gary Stormo is an American geneticist and currently Joseph Erlanger Professor in the Department of Genetics and the Center for Genome Sciences and Systems Biology at Washington University School of Medicine in St Louis. He is considered one of the pioneers of bioinformatics and genomics. His research combines experimental and computational approaches in order to identify and predict regulatory sequences in DNA and RNA, and their contributions to the regulatory networks that control gene expression.
Kimmen Sjölander is professor emerita at the University of California, Berkeley in the Department of Bioengineering. She is well known for her work on protein sequence analysis.
Non-coding RNAs have been discovered using both experimental and bioinformatic approaches. Bioinformatic approaches can be divided into three main categories. The first involves homology search, although these techniques are by definition unable to find new classes of ncRNAs. The second category includes algorithms designed to discover specific types of ncRNAs that have similar properties. Finally, some discovery methods are based on very general properties of RNA, and are thus able to discover entirely new kinds of ncRNAs.
References
- ↑ "User:Krogh - BINF - Bioinformatics Centre". Archived from the original on 2011-09-02. Retrieved 2011-04-14. Professor Anders Krogh, The Bioinformatics Centre, Department of Molecuar Biology, University of Copenhagen
- ↑ Krogh A, Brown M, Mian IS, Sjölander K, Haussler D (1994). "Hidden Markov models in computational biology. Applications to protein modeling". J. Mol. Biol. 235 (5): 1501–31. doi:10.1006/jmbi.1994.1104. PMID 8107089.
- ↑ Krogh A, Mian IS, Haussler D (1994). "A hidden Markov model that finds genes in E. coli DNA". Nucleic Acids Res. 22 (22): 4768–78. doi:10.1093/nar/22.22.4768. PMC 308529 . PMID 7984429.
- ↑ Sjölander K, Karplus K, Brown M, et al. (1996). "Dirichlet mixtures: a method for improved detection of weak but significant protein sequence homology". Comput. Appl. Biosci. 12 (4): 327–45. doi: 10.1093/bioinformatics/12.4.327 . PMID 8902360.
- ↑ Durbin, Richard M.; Eddy, Sean R.; Krogh, Anders; Mitchison, Graeme (1998), Biological Sequence Analysis: Probabilistic Models of Proteins and Nucleic Acids (1st ed.), Cambridge, New York: Cambridge University Press, ISBN 0-521-62971-3, OCLC 593254083
- ↑ Introduction to the Theory of Neural Computation (Santa Fe Institute Studies in the Sciences of Complexity). (1991) John A. Hertz, Richard G. Palmer, Anders Krogh, Westview Press
- ↑ Marstrand TT, Frellsen J, Moltke I, et al. (2008). Copley R (ed.). "Asap: A Framework for Over-Representation Statistics for Transcription Factor Binding Sites". PLOS ONE. 3 (2): e1623. Bibcode:2008PLoSO...3.1623M. doi: 10.1371/journal.pone.0001623 . PMC 2229843 . PMID 18286180.

- ↑ Frith MC, Valen E, Krogh A, Hayashizaki Y, Carninci P, Sandelin A (2008). "A code for transcription initiation in mammalian genomes". Genome Res. 18 (1): 1–12. doi:10.1101/gr.6831208. PMC 2134772 . PMID 18032727.
- ↑ Bryne JC, Valen E, Tang MH, et al. (2008). "JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update". Nucleic Acids Res. 36 (Database issue): D102–6. doi:10.1093/nar/gkm955. PMC 2238834 . PMID 18006571.
- ↑ Lindow M, Jacobsen A, Nygaard S, Mang Y, Krogh A (2007). "Intragenomic Matching Reveals a Huge Potential for miRNA-Mediated Regulation in Plants". PLOS Comput. Biol. 3 (11): e238. Bibcode:2007PLSCB...3..238L. doi: 10.1371/journal.pcbi.0030238 . PMC 2098865 . PMID 18052543.

- ↑ Lindgreen S, Gardner PP, Krogh A (2007). "MASTR: multiple alignment and structure prediction of non-coding RNAs using simulated annealing". Bioinformatics. 23 (24): 3304–11. CiteSeerX 10.1.1.563.7072 . doi:10.1093/bioinformatics/btm525. PMID 18006551.
- ↑ Lindgreen S, Gardner PP, Krogh A (2006). "Measuring covariation in RNA alignments: physical realism improves information measures". Bioinformatics. 22 (24): 2988–95. doi:10.1093/bioinformatics/btl514. PMID 17038338.
- ↑ Munch K, Krogh A (2006). "Automatic generation of gene finders for eukaryotic species". BMC Bioinformatics. 7: 263. doi: 10.1186/1471-2105-7-263 . PMC 1522026 . PMID 16712739.

- ↑ Munch K, Gardner PP, Arctander P, Krogh A (2006). "A hidden Markov model approach for determining expression from genomic tiling micro arrays". BMC Bioinformatics. 7: 239. doi: 10.1186/1471-2105-7-239 . PMC 1481622 . PMID 16672042.

- ↑ Nielsen P, Krogh A (2005). "Large-scale prokaryotic gene prediction and comparison to genome annotation". Bioinformatics. 21 (24): 4322–9. doi: 10.1093/bioinformatics/bti701 . PMID 16249266.
- ↑ Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001). "Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes". J Mol Biol. 305 (3): 567–580. doi:10.1006/jmbi.2000.4315. PMID 11152613.
- ↑ Winther O, Krogh A (2004). "Teaching computers to fold proteins". Phys. Rev. E. 70 (3): 030903. arXiv: cond-mat/0309497 . Bibcode:2004PhRvE..70c0903W. doi:10.1103/PhysRevE.70.030903. PMID 15524499. S2CID 103560.
- ↑ Won KJ, Hamelryck T, Prügel-Bennett A, Krogh A (2007). "An evolutionary method for learning HMM structure: prediction of protein secondary structure". BMC Bioinformatics. 8: 357. doi: 10.1186/1471-2105-8-357 . PMC 2072961 . PMID 17888163.

- ↑ Hamelryck T, Kent JT, Krogh A (2006). "Sampling Realistic Protein Conformations Using Local Structural Bias". PLOS Comput. Biol. 2 (9): e131. Bibcode:2006PLSCB...2..131H. doi: 10.1371/journal.pcbi.0020131 . PMC 1570370 . PMID 17002495.

- ↑ Boomsma W, Mardia KV, Taylor CC, Ferkinghoff-Borg J, Krogh A, Hamelryck T (2008). "A generative, probabilistic model of local protein structure". PNAS. 105 (26): 8932–8937. Bibcode:2008PNAS..105.8932B. doi: 10.1073/pnas.0801715105 . PMC 2440424 . PMID 18579771.
- ↑ "February 13, 2017: The International Society for Computational Biology Names Seven Members as the ISCB Fellows Class of 2017". www.iscb.org. Retrieved 13 February 2017.