
A brain tumor occurs when abnormal cells form within the brain. There are two main types of tumors: malignant tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.

A glioma is a type of tumor that starts in the glial cells of the brain or the spine. Gliomas comprise about 30 percent of all brain tumors and central nervous system tumours, and 80 percent of all malignant brain tumours.

Oligodendrogliomas are a type of glioma that are believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. They occur primarily in adults but are also found in children.

Meningioma, also known as meningeal tumor, is typically a slow-growing tumor that forms from the meninges, the membranous layers surrounding the brain and spinal cord. Symptoms depend on the location and occur as a result of the tumor pressing on nearby tissue. Many cases never produce symptoms. Occasionally seizures, dementia, trouble talking, vision problems, one sided weakness, or loss of bladder control may occur.

Glioblastoma, previously known as glioblastoma multiforme (GBM), is one of the most aggressive type of cancers that begin within the brain. Initially, signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.

Astrocytomas are a type of brain tumor. They originate in a particular kind of glial cells, star-shaped brain cells in the cerebrum called astrocytes. This type of tumor does not usually spread outside the brain and spinal cord and it does not usually affect other organs. Astrocytomas are the most common glioma and can occur in most parts of the brain and occasionally in the spinal cord.

Oligoastrocytomas are a subset of brain tumors that present with an appearance of mixed glial cell origin, astrocytoma and oligodendroglioma. However, the term "Oligoastrocytoma" is now considered obsolete by the National Comprehensive Cancer Network stating "the term should no longer be used as such morphologically ambiguous tumors can be reliably resolved into astrocytomas and oligodendrogliomas with molecular testing."

Pilocytic astrocytoma is a brain tumor that occurs most commonly in children and young adults. They usually arise in the cerebellum, near the brainstem, in the hypothalamic region, or the optic chiasm, but they may occur in any area where astrocytes are present, including the cerebral hemispheres and the spinal cord. These tumors are usually slow growing and benign, corresponding to WHO malignancy grade 1.

Temporal lobe epilepsy (TLE) is a chronic disorder of the nervous system which is characterized by recurrent, unprovoked focal seizures that originate in the temporal lobe of the brain and last about one or two minutes. TLE is the most common form of epilepsy with focal seizures. A focal seizure in the temporal lobe may spread to other areas in the brain when it may become a focal to bilateral seizure.
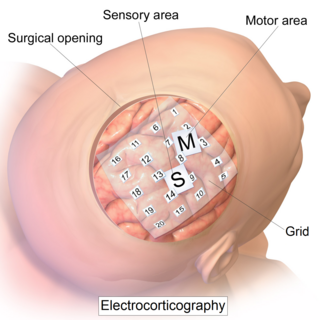
Electrocorticography (ECoG), or intracranial electroencephalography (iEEG), is a type of electrophysiological monitoring that uses electrodes placed directly on the exposed surface of the brain to record electrical activity from the cerebral cortex. In contrast, conventional electroencephalography (EEG) electrodes monitor this activity from outside the skull. ECoG may be performed either in the operating room during surgery or outside of surgery. Because a craniotomy is required to implant the electrode grid, ECoG is an invasive procedure.
Epilepsy surgery involves a neurosurgical procedure where an area of the brain involved in seizures is either resected, ablated, disconnected or stimulated. The goal is to eliminate seizures or significantly reduce seizure burden. Approximately 60% of all people with epilepsy have focal epilepsy syndromes. In 15% to 20% of these patients, the condition is not adequately controlled with anticonvulsive drugs. Such patients are potential candidates for surgical epilepsy treatment.

Dysembryoplastic neuroepithelial tumour is a type of brain tumor. Most commonly found in the temporal lobe, DNTs have been classified as benign tumours. These are glioneuronal tumours comprising both glial and neuron cells and often have ties to focal cortical dysplasia.
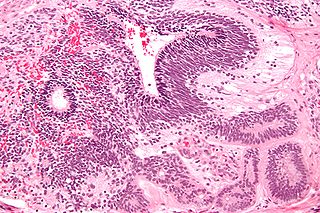
An immature teratoma is a teratoma that contains anaplastic immature elements, and is often synonymous with malignant teratoma. A teratoma is a tumor of germ cell origin, containing tissues from more than one germ cell line, It can be ovarian or testicular in its origin. and are almost always benign. An immature teratoma is thus a very rare tumor, representing 1% of all teratomas, 1% of all ovarian cancers, and 35.6% of malignant ovarian germ cell tumors. It displays a specific age of incidence, occurring most frequently in the first two decades of life and almost never after menopause. Unlike a mature cystic teratoma, an immature teratoma contains immature or embryonic structures. It can coexist with mature cystic teratomas and can constitute of a combination of both adult and embryonic tissue. The most common symptoms noted are abdominal distension and masses. Prognosis and treatment options vary and largely depend on grade, stage and karyotype of the tumor itself.
Pleomorphic xanthoastrocytoma (PXA) is a brain tumor that occurs most frequently in children and teenagers. At Boston Children's Hospital, the average age at diagnosis is 12 years.
Neuro-oncology is the study of brain and spinal cord neoplasms, many of which are very dangerous and life-threatening. Among the malignant brain cancers, gliomas of the brainstem and pons, glioblastoma multiforme, and high-grade astrocytoma are among the worst. In these cases, untreated survival usually amounts to only a few months, and survival with current radiation and chemotherapy treatments may extend that time from around a year to a year and a half, possibly two or more, depending on the patient's condition, immune function, treatments used, and the specific type of malignant brain neoplasm. Surgery may in some cases be curative, but, as a general rule, malignant brain cancers tend to regenerate and emerge from remission easily, especially highly malignant cases. In such cases, the goal is to excise as much of the mass and as much of the tumor margin as possible without endangering vital functions or other important cognitive abilities. The Journal of Neuro-Oncology is the longest continuously published journal in the field and serves as a leading reference to those practicing in the area of neuro-oncology.

Tuber cinereum hamartoma is a benign tumor in which a disorganized collection of neurons and glia accumulate at the tuber cinereum of the hypothalamus on the floor of the third ventricle. It is a congenital malformation, included on the spectrum of gray matter heterotopias. Formation occurs during embryogenesis, typically between days 33 and 41 of gestation. Size of the tumor varies from one to three centimeters in diameter, with the mean being closer to the low end of this range. It is estimated to occur at a frequency of one in one million individuals.
Cortical stimulation mapping (CSM) is a type of electrocorticography that involves a physically invasive procedure and aims to localize the function of specific brain regions through direct electrical stimulation of the cerebral cortex. It remains one of the earliest methods of analyzing the brain and has allowed researchers to study the relationship between cortical structure and systemic function. Cortical stimulation mapping is used for a number of clinical and therapeutic applications, and remains the preferred method for the pre-surgical mapping of the motor cortex and language areas to prevent unnecessary functional damage. There are also some clinical applications for cortical stimulation mapping, such as the treatment of epilepsy.

Low-grade fibromyxoid sarcoma (LGFMS) is a rare type of low-grade sarcoma first described by H. L. Evans in 1987. LGFMS are soft tissue tumors of the mesenchyme-derived connective tissues; on microscopic examination, they are found to be composed of spindle-shaped cells that resemble fibroblasts. These fibroblastic, spindle-shaped cells are neoplastic cells that in most cases of LGFMS express fusion genes, i.e. genes composed of parts of two different genes that form as a result of mutations. The World Health Organization (2020) classified LGFMS as a specific type of tumor in the category of malignant fibroblastic and myofibroblastic tumors.

Astroblastoma is a rare glial tumor derived from the astroblast, a type of cell that closely resembles spongioblastoma and astrocytes. Astroblastoma cells are most likely found in the supratentorial region of the brain that houses the cerebrum, an area responsible for all voluntary movements in the body. It also occurs significantly in the frontal lobe, parietal lobe, and temporal lobe, areas where movement, language creation, memory perception, and environmental surroundings are expressed. These tumors can be present in major brain areas not associated with the main cerebral hemispheres, including the cerebellum, optic nerve, cauda equina, hypothalamus, and brain stem.
Myxofibrosarcoma (MFS), although a rare type of tumor, is one of the most common soft tissue sarcomas, i.e. cancerous tumors, that develop in the soft tissues of elderly individuals. Initially considered to be a type of histiocytoma termed fibrous histiocytoma or myxoid variant of malignant fibrous histiocytoma, Angervall et al. termed this tumor myxofibrosarcoma in 1977. In 2020, the World Health Organization reclassified MFS as a separate and distinct tumor in the category of malignant fibroblastic and myofibroblastic tumors.




















