
The striatum or corpus striatum is a nucleus in the subcortical basal ganglia of the forebrain. The striatum is a critical component of the motor and reward systems; receives glutamatergic and dopaminergic inputs from different sources; and serves as the primary input to the rest of the basal ganglia.
The amygdala is a paired nuclear complex present in the cerebral hemispheres of vertebrates. It is considered part of the limbic system. In primates, it is located medially within the temporal lobes. It consists of many nuclei, each made up of further subnuclei. The subdivision most commonly made is into the basolateral, central, cortical, and medial nuclei together with the intercalated cell clusters. The amygdala has a primary role in the processing of memory, decision-making, and emotional responses. The amygdala was first identified and named by Karl Friedrich Burdach in 1822.
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventral striatum includes the nucleus accumbens and the olfactory tubercle.

Pavlovian fear conditioning is a behavioral paradigm in which organisms learn to predict aversive events. It is a form of learning in which an aversive stimulus is associated with a particular neutral context or neutral stimulus, resulting in the expression of fear responses to the originally neutral stimulus or context. This can be done by pairing the neutral stimulus with an aversive stimulus. Eventually, the neutral stimulus alone can elicit the state of fear. In the vocabulary of classical conditioning, the neutral stimulus or context is the "conditional stimulus" (CS), the aversive stimulus is the "unconditional stimulus" (US), and the fear is the "conditional response" (CR).

The nucleus accumbens is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia. The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle. Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
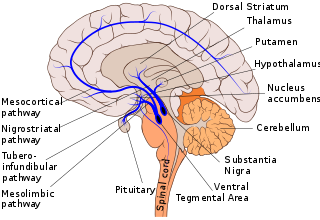
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.

The ventral tegmental area (VTA), also known as the ventral tegmental area of Tsai, or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine pathways; it is widely implicated in the drug and natural reward circuitry of the brain. The VTA plays an important role in a number of processes, including reward cognition and orgasm, among others, as well as several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, ranging from the prefrontal cortex to the caudal brainstem and several regions in between.
Motivational salience is a cognitive process and a form of attention that motivates or propels an individual's behavior towards or away from a particular object, perceived event or outcome. Motivational salience regulates the intensity of behaviors that facilitate the attainment of a particular goal, the amount of time and energy that an individual is willing to expend to attain a particular goal, and the amount of risk that an individual is willing to accept while working to attain a particular goal.
An avoidance response is a natural adaptive behavior performed in response to danger. Excessive avoidance has been suggested to contribute to anxiety disorders, leading psychologists and neuroscientists to study how avoidance behaviors are learned using rat or mouse models. Avoidance learning is a type of operant conditioning.
The amygdalofugal pathway is one of the three major efferent pathways of the amygdala, meaning that it is one of the three principal pathways by which fibers leave the amygdala. It leads from the basolateral nucleus and central nucleus of the amygdala. The amygdala is a limbic structure in the medial temporal lobe of the brain. The other main efferent pathways from the amygdala are the stria terminalis and anterior commissure.

Medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), are a special type of GABAergic inhibitory cell representing 95% of neurons within the human striatum, a basal ganglia structure. Medium spiny neurons have two primary phenotypes : D1-type MSNs of the direct pathway and D2-type MSNs of the indirect pathway. Most striatal MSNs contain only D1-type or D2-type dopamine receptors, but a subpopulation of MSNs exhibit both phenotypes.

The reward system is a group of neural structures responsible for incentive salience, associative learning, and positively-valenced emotions, particularly ones involving pleasure as a core component. Reward is the attractive and motivational property of a stimulus that induces appetitive behavior, also known as approach behavior, and consummatory behavior. A rewarding stimulus has been described as "any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward". In operant conditioning, rewarding stimuli function as positive reinforcers; however, the converse statement also holds true: positive reinforcers are rewarding.
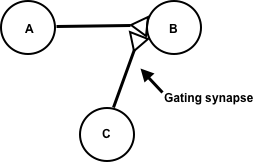
Synaptic gating is the ability of neural circuits to gate inputs by either suppressing or facilitating specific synaptic activity. Selective inhibition of certain synapses has been studied thoroughly, and recent studies have supported the existence of permissively gated synaptic transmission. In general, synaptic gating involves a mechanism of central control over neuronal output. It includes a sort of gatekeeper neuron, which has the ability to influence transmission of information to selected targets independently of the parts of the synapse upon which it exerts its action.
The Intercalatedcells of the amygdala are GABAergic neurons situated between the basolateral and central nuclei of the amygdala that play a significant role in inhibitory control over the amygdala. They regulate amygdala-dependent emotional processing like fear memory and social behavior. Their function has been best studied with selective ITC ablation which impairs fear extinction, fear generalization, and social behavior. Studies have begun to recognize that ITC clusters may be implicated in reward, addiction, and withdrawal circuits given their heavy expression of dopamine and opioid receptors.
Many experiments have been done to find out how the brain interprets stimuli and how animals develop fear responses. The emotion, fear, has been hard-wired into almost every individual, due to its vital role in the survival of the individual. Researchers have found that fear is established unconsciously and that the amygdala is involved with fear conditioning.
Addiction is a state characterized by compulsive engagement in rewarding stimuli, despite adverse consequences. The process of developing an addiction occurs through instrumental learning, which is otherwise known as operant conditioning.
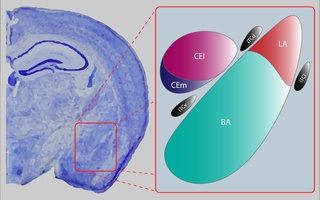
The central nucleus of the amygdala is a nucleus within the amygdala. It "serves as the major output nucleus of the amygdala and participates in receiving and processing pain information."
Pavlovian-instrumental transfer (PIT) is a psychological phenomenon that occurs when a conditioned stimulus that has been associated with rewarding or aversive stimuli via classical conditioning alters motivational salience and operant behavior. Two distinct forms of Pavlovian-instrumental transfer have been identified in humans and other animals – specific PIT and general PIT – with unique neural substrates mediating each type. In relation to rewarding stimuli, specific PIT occurs when a CS is associated with a specific rewarding stimulus through classical conditioning and subsequent exposure to the CS enhances an operant response that is directed toward the same reward with which it was paired. General PIT occurs when a CS is paired with one reward and it enhances an operant response that is directed toward a different rewarding stimulus.

Kate Wassum is an American neuroscientist and professor of behavioral neuroscience at the University of California, Los Angeles. Wassum probes the neural circuits underlying appetitive associative learning the circuit dynamics that give rise to diverse motivated behaviors.
Fan Wang is a neuroscientist and professor in the MIT Department of Brain and Cognitive Sciences. She is an investigator at the McGovern Institute for Brain Research. Wang is known for her work identifying neural circuits underlying touch, pain, and anesthesia; and the development of a technique for capturing activated neuronal ensembles (CANE) to label and manipulate neurons activated by stimuli or behavioral paradigms.











