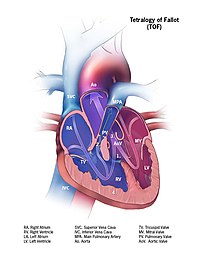
Tetralogy of Fallot (TOF), formerly known as Steno-Fallot tetralogy, is a congenital heart defect characterized by four specific cardiac defects. Classically, the four defects are:

Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications may include seizures and heart arrhythmias.

Cyanosis is the change of body tissue color to a bluish-purple hue, as a result of decrease in the amount of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Cyanosis is apparent usually in the body tissues covered with thin skin, including the mucous membranes, lips, nail beds, and ear lobes. Some medications may cause discoloration such as medications containing amiodarone or silver. Furthermore, mongolian spots, large birthmarks, and the consumption of food products with blue or purple dyes can also result in the bluish skin tissue discoloration and may be mistaken for cyanosis. Appropriate physical examination and history taking is a crucial part to diagnose cyanosis. Management of cyanosis involves treating the main cause, as cyanosis isn’t a disease, it is a symptom.
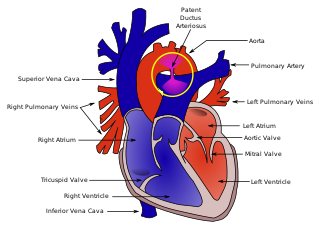
Patent ductus arteriosus (PDA) is a medical condition in which the ductus arteriosus fails to close after birth: this allows a portion of oxygenated blood from the left heart to flow back to the lungs through the aorta, which has a higher blood pressure, to the pulmonary artery, which has a lower blood pressure. Symptoms are uncommon at birth and shortly thereafter, but later in the first year of life there is often the onset of an increased work of breathing and failure to gain weight at a normal rate. With time, an uncorrected PDA usually leads to pulmonary hypertension followed by right-sided heart failure.

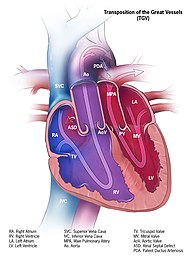
dextro-Transposition of the great arteries is a potentially life-threatening birth defect in the large arteries of the heart. The primary arteries are transposed.

A congenital heart defect (CHD), also known as a congenital heart anomaly, congenital cardiovascular malformation, and congenital heart disease, is a defect in the structure of the heart or great vessels that is present at birth. A congenital heart defect is classed as a cardiovascular disease. Signs and symptoms depend on the specific type of defect. Symptoms can vary from none to life-threatening. When present, symptoms are variable and may include rapid breathing, bluish skin (cyanosis), poor weight gain, and feeling tired. CHD does not cause chest pain. Most congenital heart defects are not associated with other diseases. A complication of CHD is heart failure.
A cyanotic heart defect is any congenital heart defect (CHD) that occurs due to deoxygenated blood bypassing the lungs and entering the systemic circulation, or a mixture of oxygenated and unoxygenated blood entering the systemic circulation. It is caused by structural defects of the heart such as right-to-left or bidirectional shunting, malposition of the great arteries, or any condition which increases pulmonary vascular resistance. The result may be the development of collateral circulation.

Methemoglobin (British: methaemoglobin, shortened MetHb) (pronounced "met-hemoglobin") is a hemoglobin in the form of metalloprotein, in which the iron in the heme group is in the Fe3+ (ferric) state, not the Fe2+ (ferrous) of normal hemoglobin. Sometimes, it is also referred to as ferrihemoglobin. Methemoglobin cannot bind oxygen, which means it cannot carry oxygen to tissues. It is bluish chocolate-brown in color. In human blood a trace amount of methemoglobin is normally produced spontaneously, but when present in excess the blood becomes abnormally dark bluish brown. The NADH-dependent enzyme methemoglobin reductase (a type of diaphorase) is responsible for converting methemoglobin back to hemoglobin.

Eisenmenger syndrome or Eisenmenger's syndrome is defined as the process in which a long-standing left-to-right cardiac shunt caused by a congenital heart defect causes pulmonary hypertension and eventual reversal of the shunt into a cyanotic right-to-left shunt. Because of the advent of fetal screening with echocardiography early in life, the incidence of heart defects progressing to Eisenmenger syndrome has decreased.

The Blalock–Thomas–Taussig shunt, previously known as the Blalock-Taussig Shunt, is a surgical procedure used to increase blood flow to the lungs in some forms of congenital heart disease such as pulmonary atresia and tetralogy of Fallot, which are common causes of blue baby syndrome. The procedure involves connecting a branch of the subclavian artery or carotid artery to the pulmonary artery. In modern practice, this procedure is temporarily used to direct blood flow to the lungs and relieve cyanosis while the infant is waiting for corrective or definitive surgery when their heart is larger. The BTT shunt is used in the first step of the three-stage palliation.

Helen Brooke Taussig was an American cardiologist, working in Baltimore and Boston, who founded the field of pediatric cardiology. She is credited with developing the concept for a procedure that would extend the lives of children born with Tetralogy of Fallot. This concept was applied in practice as a procedure known as the Blalock-Thomas-Taussig shunt. The procedure was developed by Alfred Blalock and Vivien Thomas, who were Taussig's colleagues at the Johns Hopkins Hospital.
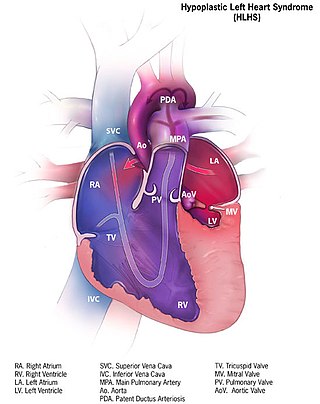
Hypoplastic left heart syndrome (HLHS) is a rare congenital heart defect in which the left side of the heart is severely underdeveloped and incapable of supporting the systemic circulation. It is estimated to account for 2-3% of all congenital heart disease. Early signs and symptoms include poor feeding, cyanosis, and diminished pulse in the extremities. The etiology is believed to be multifactorial resulting from a combination of genetic mutations and defects resulting in altered blood flow in the heart. Several structures can be affected including the left ventricle, aorta, aortic valve, or mitral valve all resulting in decreased systemic blood flow.

Transposition of the great vessels (TGV) is a group of congenital heart defects involving an abnormal spatial arrangement of any of the great vessels: superior and/or inferior venae cavae, pulmonary artery, pulmonary veins, and aorta. Congenital heart diseases involving only the primary arteries belong to a sub-group called transposition of the great arteries (TGA), which is considered the most common congenital heart lesion that presents in neonates.

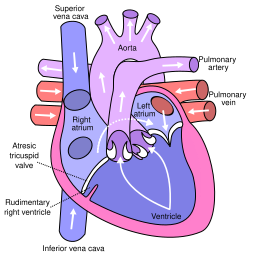
Tricuspid atresia is a form of congenital heart disease whereby there is a complete absence of the tricuspid valve. Therefore, there is an absence of right atrioventricular connection. This leads to a hypoplastic (undersized) or absent right ventricle. This defect is contracted during prenatal development, when the heart does not finish developing. It causes the systemic circulation to be filled with relatively deoxygenated blood. The causes of tricuspid atresia are unknown.

Atrioventricular septal defect (AVSD) or atrioventricular canal defect (AVCD), also known as "common atrioventricular canal" or "endocardial cushion defect" (ECD), is characterized by a deficiency of the atrioventricular septum of the heart that creates connections between all four of its chambers. It is a very specific combination of 3 defects:
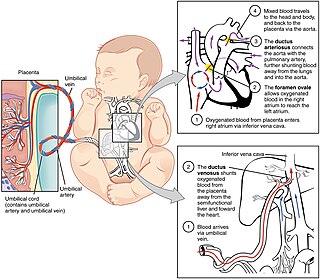
In humans, the circulatory system is different before and after birth. The fetal circulation is composed of the placenta, umbilical blood vessels encapsulated by the umbilical cord, heart and systemic blood vessels. A major difference between the fetal circulation and postnatal circulation is that the lungs are not used during the fetal stage resulting in the presence of shunts to move oxygenated blood and nutrients from the placenta to the fetal tissue. At birth, the start of breathing and the severance of the umbilical cord prompt various changes that quickly transform fetal circulation into postnatal circulation.
A right-to-left shunt is a cardiac shunt which allows blood to flow from the right heart to the left heart. This terminology is used both for the abnormal state in humans and for normal physiological shunts in reptiles.
Hypoplastic right heart syndrome or HRHS is a congenital heart defect in which the structures on the right side of the heart, particularly the right ventricle, are underdeveloped. This defect causes inadequate blood flow to the lungs, and thus a cyanotic infant.

Hemoglobin M disease is a rare form of hemoglobinopathy, characterized by the presence of hemoglobin M (HbM) and elevated methemoglobin (metHb) level in blood. HbM is an altered form of hemoglobin (Hb) due to point mutation occurring in globin-encoding genes, mostly involving tyrosine substitution for proximal (F8) or distal (E7) histidine residues. HbM variants are inherited as autosomal dominant disorders and have altered oxygen affinity. The pathophysiology of hemoglobin M disease involves heme iron autoxidation promoted by heme pocket structural alteration.
Raghib syndrome is rare a congenital heart defect where the left superior vena cava (LSVC) is draining into the left atrium in addition to an absent coronary sinus and an atrial septal defect. This can be considered a dangerous heart condition because it puts the individual at a high risk of stroke. Other defects that are often associated with Raghib syndrome can include ventricular septal defects, enlargement of the tricuspid annulus, and pulmonary stenosis. While this is considered an extremely rare developmental complex, cases regarding a persistent left superior vena cava (PLSVC) are relatively common among congenital heart defects. It is also important to note that the PLSVC often drains into the right atrium, and only drains into the left atrium in approximately 10 to 20% of individuals with the defect.