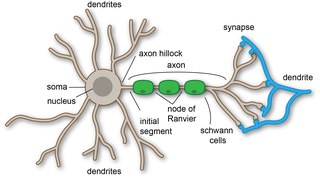
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.
Spike-timing-dependent plasticity (STDP) is a biological process that adjusts the strength of connections between neurons in the brain. The process adjusts the connection strengths based on the relative timing of a particular neuron's output and input action potentials. The STDP process partially explains the activity-dependent development of nervous systems, especially with regard to long-term potentiation and long-term depression.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.

Purkinje cells or Purkinje neurons, named for Czech physiologist Jan Evangelista Purkyně who identified them in 1837, are a unique type of prominent large neurons located in the cerebellar cortex of the brain. With their flask-shaped cell bodies, many branching dendrites, and a single long axon, these cells are essential for controlling motor activity. Purkinje cells mainly release GABA neurotransmitter, which inhibits some neurons to reduce nerve impulse transmission. Purkinje cells efficiently control and coordinate the body's motor motions through these inhibitory actions.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

Neural oscillations, or brainwaves, are rhythmic or repetitive patterns of neural activity in the central nervous system. Neural tissue can generate oscillatory activity in many ways, driven either by mechanisms within individual neurons or by interactions between neurons. In individual neurons, oscillations can appear either as oscillations in membrane potential or as rhythmic patterns of action potentials, which then produce oscillatory activation of post-synaptic neurons. At the level of neural ensembles, synchronized activity of large numbers of neurons can give rise to macroscopic oscillations, which can be observed in an electroencephalogram. Oscillatory activity in groups of neurons generally arises from feedback connections between the neurons that result in the synchronization of their firing patterns. The interaction between neurons can give rise to oscillations at a different frequency than the firing frequency of individual neurons. A well-known example of macroscopic neural oscillations is alpha activity.

Neuronal noise or neural noise refers to the random intrinsic electrical fluctuations within neuronal networks. These fluctuations are not associated with encoding a response to internal or external stimuli and can be from one to two orders of magnitude. Most noise commonly occurs below a voltage-threshold that is needed for an action potential to occur, but sometimes it can be present in the form of an action potential; for example, stochastic oscillations in pacemaker neurons in suprachiasmatic nucleus are partially responsible for the organization of circadian rhythms.
Neural coding is a neuroscience field concerned with characterising the hypothetical relationship between the stimulus and the neuronal responses, and the relationship among the electrical activities of the neurons in the ensemble. Based on the theory that sensory and other information is represented in the brain by networks of neurons, it is believed that neurons can encode both digital and analog information.
In neuroscience, homeostatic plasticity refers to the capacity of neurons to regulate their own excitability relative to network activity. The term homeostatic plasticity derives from two opposing concepts: 'homeostatic' and plasticity, thus homeostatic plasticity means "staying the same through change". In the nervous system, neurons must be able to evolve with the development of their constantly changing environment while simultaneously staying the same amidst this change. This stability is important for neurons to maintain their activity and functionality to prevent neurons from carcinogenesis. At the same time, neurons need to have flexibility to adapt to changes and make connections to cope with the ever-changing environment of a developing nervous system.
The preBötzinger complex, often abbreviated as preBötC, is a functionally and anatomically specialized site in the ventral-lateral region of the lower medulla oblongata. The preBötC is part of the ventral respiratory group of respiratory related interneurons. Its foremost function is to generate the inspiratory breathing rhythm in mammals. In addition, the preBötC is widely and paucisynaptically connected to higher brain centers that regulate arousal and excitability more generally such that respiratory brain function is intimately connected with many other rhythmic and cognitive functions of the brain and central nervous system. Further, the preBötC receives mechanical sensory information from the airways that encode lung volume as well as pH, oxygen, and carbon dioxide content of circulating blood and the cerebrospinal fluid.
Neural backpropagation is the phenomenon in which, after the action potential of a neuron creates a voltage spike down the axon, another impulse is generated from the soma and propagates towards the apical portions of the dendritic arbor or dendrites. In addition to active backpropagation of the action potential, there is also passive electrotonic spread. While there is ample evidence to prove the existence of backpropagating action potentials, the function of such action potentials and the extent to which they invade the most distal dendrites remain highly controversial.

Biological neuron models, also known as spiking neuron models, are mathematical descriptions of the conduction of electrical signals in neurons. Neurons are electrically excitable cells within the nervous system, able to fire electric signals, called action potentials, across a neural network.These mathematical models describe the role of the biophysical and geometrical characteristics of neurons on the conduction of electrical activity.

Subthreshold membrane potential oscillations are membrane oscillations that do not directly trigger an action potential since they do not reach the necessary threshold for firing. However, they may facilitate sensory signal processing.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
Developmental plasticity is a general term referring to changes in neural connections during development as a result of environmental interactions as well as neural changes induced by learning. Much like neuroplasticity, or brain plasticity, developmental plasticity is specific to the change in neurons and synaptic connections as a consequence of developmental processes. A child creates most of these connections from birth to early childhood. There are three primary methods by which this may occur as the brain develops, but critical periods determine when lasting changes may form. Developmental plasticity may also be used in place of the term phenotypic plasticity when an organism in an embryonic or larval stage can alter its phenotype based on environmental factors. However, a main difference between the two is that phenotypic plasticity experienced during adulthood can be reversible, whereas traits that are considered developmentally plastic set foundations during early development that remain throughout the life of the organism.

The theta model, or Ermentrout–Kopell canonical model, is a biological neuron model originally developed to mathematically describe neurons in the animal Aplysia. The model is particularly well-suited to describe neural bursting, which is characterized by periodic transitions between rapid oscillations in the membrane potential followed by quiescence. This bursting behavior is often found in neurons responsible for controlling and maintaining steady rhythms such as breathing, swimming, and digesting. Of the three main classes of bursting neurons, the theta model describes parabolic bursting, which is characterized by a parabolic frequency curve during each burst.

Eberhard Erich Fetz is an American neuroscientist, academic and researcher. He is a Professor of Physiology and Biophysics and DXARTS at the University of Washington.
Rinzel J. (1986) A formal Classification of Bursting Mechanisms in Excitable Systems. Proceedings of the International Congress of Mathematicians. Berkeley, California, USA



















