Related Research Articles

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

A protease is an enzyme that catalyzes proteolysis, the breakdown of proteins into smaller polypeptides or single amino acids. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.
A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example of this would be ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.
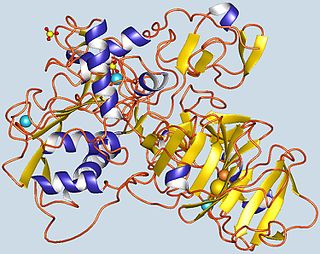
Gelatinase A, also known as MMP2 is an enzyme. This enzyme catalyses the following chemical reaction
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:
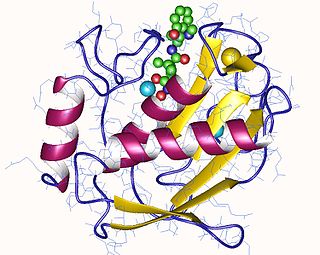
Interstitial collagenase is an enzyme. This enzyme catalyses the following chemical reaction
Microbial collagenase is an enzyme. This enzyme catalyses the following chemical reaction

ADAMs are a family of single-pass transmembrane and secreted metalloendopeptidases. All ADAMs are characterized by a particular domain organization featuring a pro-domain, a metalloprotease, a disintegrin, a cysteine-rich, an epidermal-growth factor like and a transmembrane domain, as well as a C-terminal cytoplasmic tail. Nonetheless, not all human ADAMs have a functional protease domain, which indicates that their biological function mainly depends on protein–protein interactions. Those ADAMs which are active proteases are classified as sheddases because they cut off or shed extracellular portions of transmembrane proteins. For example, ADAM10 can cut off part of the HER2 receptor, thereby activating it. ADAM genes are found in animals, choanoflagellates, fungi and some groups of green algae. Most green algae and all land plants likely lost ADAM proteins.
In molecular biology, the Signal Peptide Peptidase (SPP) is a type of protein that specifically cleaves parts of other proteins. It is an intramembrane aspartyl protease with the conserved active site motifs 'YD' and 'GxGD' in adjacent transmembrane domains (TMDs). Its sequences is highly conserved in different vertebrate species. SPP cleaves remnant signal peptides left behind in membrane by the action of signal peptidase and also plays key roles in immune surveillance and the maturation of certain viral proteins.

Clostridium perfringens alpha toxin is a toxin produced by the bacterium Clostridium perfringens and is responsible for gas gangrene and myonecrosis in infected tissues. The toxin also possesses hemolytic activity.

Matrilysin also known as matrix metalloproteinase-7 (MMP-7), pump-1 protease (PUMP-1), or uterine metalloproteinase is an enzyme in humans that is encoded by the MMP7 gene. The enzyme has also been known as matrin, putative metalloproteinase-1, matrix metalloproteinase pump 1, PUMP-1 proteinase, PUMP, metalloproteinase pump-1, putative metalloproteinase, MMP).

A disintegrin and metalloproteinase with thrombospondin motifs 10 is an enzyme that in humans is encoded by the ADAMTS10 gene.

Neutrophil collagenase, also known as matrix metalloproteinase-8 (MMP-8) or PMNL collagenase (MNL-CL), is a collagen cleaving enzyme which is present in the connective tissue of most mammals. In humans, the MMP-8 protein is encoded by the MMP8 gene. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. However, the enzyme encoded by this gene is stored in secondary granules within neutrophils and is activated by autolytic cleavage.

Ecadotril is a neutral endopeptidase inhibitor and determined by the presence of peptidase family M13 as a neutral endopeptidase inhibited by phosphoramidon. Ecadotril is the (S)-enantiomer of racecadotril. NEP-like enzymes include the endothelin-converting enzymes. The peptidase M13 family believed to activate or inactivate oligopeptide (pro)-hormones such as opioid peptides, neprilysin is another member of this group, in the case of the metallopeptidases and aspartic, the nucleophiles clan or family for example MA, is an activated water molecule. The peptidase domain for members of this family also contains a bacterial member and resembles that of thermolysin the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. Thermolysin complexed with the inhibitor (S)-thiorphan are isomeric thiol-containing inhibitors of endopeptidase EC 24-11.
Clostridium histolyticum is a species of bacteria found in feces and the soil. It is a motile, gram-positive, aerotolerant anaerobe. C. histolyticum is pathogenic in many species, including guinea pigs, mice, and rabbits, and humans. C. histolyticum has been shown to cause gas gangrene, often in association with other bacteria species.

Astacins are a family of multidomain metalloendopeptidases which are either secreted or membrane-anchored. These metallopeptidases belong to the MEROPS peptidase family M12, subfamily M12A. The protein fold of the peptidase domain for members of this family resembles that of thermolysin, the type example for clan MA and the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH.

Zingibain, zingipain, or ginger protease is a cysteine protease enzyme found in ginger rhizomes. It catalyses the preferential cleavage of peptides with a proline residue at the P2 position. It has two distinct forms, ginger protease I (GP-I) and ginger protease II (GP-II).
Pseudolysin is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Gerard J. Tortora; Berdell R. Funke; Cristine L. Case (2007). Microbiology: an introduction. Pearson Benjamin Cummings. ISBN 978-0-321-39603-7.
- ↑ Riley KN, Herman IM (2005). "Collagenase promotes the cellular responses to injury and wound healing in vivo". J Burns Wounds. 4: e8. PMC 1501117 . PMID 16921413.
- 1 2 3 Rawlings ND, Barrett AJ (1995). "Evolutionary families of metallopeptidases". Meth. Enzymol. 248: 183–228. doi:10.1016/0076-6879(95)48015-3. PMID 7674922.
- 1 2 Matsushita O, Yoshihara K, Katayama S, Minami J, Okabe A (January 1994). "Purification and characterization of Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene". J. Bacteriol. 176 (1): 149–56. doi:10.1128/jb.176.1.149-156.1994. PMC 205026 . PMID 8282691.
- ↑ Zhao, Guo-Yan; Zhou, Ming-Yang; Zhao, Hui-Lin; Chen, Xiu-Lan; Xie, Bin-Bin; Zhang, Xi-Ying; He, Hai-Lun; Zhou, Bai-Cheng; Zhang, Yu-Zhong (2012-10-15). "Tenderization effect of cold-adapted collagenolytic protease MCP-01 on beef meat at low temperature and its mechanism". Food Chemistry. 134 (4): 1738–1744. doi:10.1016/j.foodchem.2012.03.118. ISSN 0308-8146. PMID 23442615.