Related Research Articles

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

The pulp is the connective tissue, nerves, blood vessels, and odontoblasts that comprise the innermost layer of a tooth. The pulp's activity and signalling processes regulate its behaviour.

Ameloblasts are cells present only during tooth development that deposit tooth enamel, which is the hard outermost layer of the tooth forming the surface of the crown.

Deciduous teeth or primary teeth, also informally known as baby teeth, milk teeth, or temporary teeth, are the first set of teeth in the growth and development of humans and other diphyodonts, which include most mammals but not elephants, kangaroos, or manatees, which are polyphyodonts. Deciduous teeth develop during the embryonic stage of development and erupt during infancy. They are usually lost and replaced by permanent teeth, but in the absence of their permanent replacements, they can remain functional for many years into adulthood.

The periodontal ligament, commonly abbreviated as the PDL, is a group of specialized connective tissue fibers that essentially attach a tooth to the alveolar bone within which it sits. It inserts into root cementum on one side and onto alveolar bone on the other.
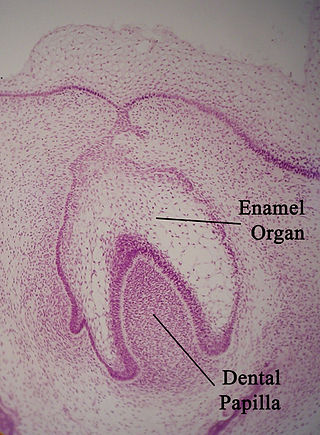
The enamel organ, also known as the dental organ, is a cellular aggregation seen in a developing tooth and it lies above the dental papilla. The enamel organ which is differentiated from the primitive oral epithelium lining the stomodeum. The enamel organ is responsible for the formation of enamel, initiation of dentine formation, establishment of the shape of a tooth's crown, and establishment of the dentoenamel junction.

In vertebrates, an odontoblast is a cell of neural crest origin that is part of the outer surface of the dental pulp, and whose biological function is dentinogenesis, which is the formation of dentin, the substance beneath the tooth enamel on the crown and the cementum on the root.

Regenerative medicine deals with the "process of replacing, engineering or regenerating human or animal cells, tissues or organs to restore or establish normal function". This field holds the promise of engineering damaged tissues and organs by stimulating the body's own repair mechanisms to functionally heal previously irreparable tissues or organs.

The dental follicle, also known as dental sac, is made up of mesenchymal cells and fibres surrounding the enamel organ and dental papilla of a developing tooth. It is a vascular fibrous sac containing the developing tooth and its odontogenic organ. The dental follicle (DF) differentiates into the periodontal ligament. In addition, it may be the precursor of other cells of the periodontium, including osteoblasts, cementoblasts and fibroblasts. They develop into the alveolar bone, the cementum with Sharpey's fibers and the periodontal ligament fibers respectively. Similar to dental papilla, the dental follicle provides nutrition to the enamel organ and dental papilla and also have an extremely rich blood supply.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine.
Stem-cell therapy is the use of stem cells to treat or prevent a disease or condition. As of 2016, the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.

Nestin is a protein that in humans is encoded by the NES gene.

Transcription factor Sp7, also called osterix (Osx), is a protein that in humans is encoded by the SP7 gene. It is a member of the Sp family of zinc-finger transcription factors It is highly conserved among bone-forming vertebrate species It plays a major role, along with Runx2 and Dlx5 in driving the differentiation of mesenchymal precursor cells into osteoblasts and eventually osteocytes. Sp7 also plays a regulatory role by inhibiting chondrocyte differentiation maintaining the balance between differentiation of mesenchymal precursor cells into ossified bone or cartilage. Mutations of this gene have been associated with multiple dysfunctional bone phenotypes in vertebrates. During development, a mouse embryo model with Sp7 expression knocked out had no formation of bone tissue. Through the use of GWAS studies, the Sp7 locus in humans has been strongly associated with bone mass density. In addition there is significant genetic evidence for its role in diseases such as Osteogenesis imperfecta (OI).

Resorption of the root of the tooth, or root resorption, is the progressive loss of dentin and cementum by the action of odontoclasts. Root resorption is a normal physiological process that occurs in the exfoliation of the primary dentition. However, pathological root resorption occurs in the permanent or secondary dentition and sometimes in the primary dentition.

Mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells or medicinal signaling cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes and adipocytes.
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.

Regenerative endodontic procedures is defined as biologically based procedures designed to replace damaged structures such as dentin, root structures, and cells of the pulp-dentin complex. This new treatment modality aims to promote normal function of the pulp. It has become an alternative to heal apical periodontitis. Regenerative endodontics is the extension of root canal therapy. Conventional root canal therapy cleans and fills the pulp chamber with biologically inert material after destruction of the pulp due to dental caries, congenital deformity or trauma. Regenerative endodontics instead seeks to replace live tissue in the pulp chamber. The ultimate goal of regenerative endodontic procedures is to regenerate the tissues and the normal function of the dentin-pulp complex.
Craniofacial regeneration refers to the biological process by which the skull and face regrow to heal an injury. This page covers birth defects and injuries related to the craniofacial region, the mechanisms behind the regeneration, the medical application of these processes, and the scientific research conducted on this specific regeneration. This regeneration is not to be confused with tooth regeneration. Craniofacial regrowth is broadly related to the mechanisms of general bone healing.
Professor Alastair J Sloan is an applied bioscientist and expert in the broad field of mineralised connective tissues, and since January 2020 current head of the Melbourne Dental School, University of Melbourne.
References
- ↑ Huang, G. T.; Gronthos, S.; Shi, S. (2009). "Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources". Journal of Dental Research. 88 (9): 792–806. doi:10.1177/0022034509340867. PMC 2830488 . PMID 19767575.
- 1 2 3 Atari, M.; Gil-Recio, C.; Fabregat, M.; García-Fernández, D.; Barajas, M.; Carrasco, M. A.; Jung, H. S.; Alfaro, F. H.; Casals, N.; Prosper, F.; Ferrés-Padró, E.; Giner, L. (2012). "Dental pulp of the third molar: A new source of pluripotent-like stem cells". Journal of Cell Science. 125 (Pt 14): 3343–56. doi: 10.1242/jcs.096537 . PMID 22467856.
- ↑ Gronthos, S.; Brahim, J.; Li, W.; Fisher, L. W.; Cherman, N.; Boyde, A.; Denbesten, P.; Robey, P. G.; Shi, S. (2002). "Stem cell properties of human dental pulp stem cells". Journal of Dental Research. 81 (8): 531–5. doi:10.1177/154405910208100806. PMID 12147742. S2CID 8921170.
- ↑ Labedz-Maslowska, A.; Bryniarska, N.; Kubiak, A.; Kaczmarzyk, T.; Sekula-Stryjewska, M.; Noga, S.; Boruczkowski, D.; Madeja, Z.; Zuba-Surma, E. (2020). "Multilineage Differentiation Potential of Human Dental Pulp Stem Cells-Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro". Int J Mol Sci. 21 (17): 6172. doi: 10.3390/ijms21176172 . PMC 7504399 . PMID 32859105.
- ↑ Morsczeck, C.; Reichert, T. E. (2018). "Dental stem cells in tooth regeneration and repair in the future". Expert Opinion on Biological Therapy. 18 (2): 187–196. doi:10.1080/14712598.2018.1402004. PMID 29110535. S2CID 41147569.
- ↑ Volponi, A. A. (2010). "Stem cell-based biological tooth repair and regeneration". Trends in Cell Biology. 20 (12): 715–722. doi:10.1016/j.tcb.2010.09.012. PMC 3000521 . PMID 21035344.
- 1 2 3 4 Song, D.; Xu, P.; Liu, S.; Wu, S. (2019). "Dental pulp stem cells expressing SIRT1 improve new bone formation during distraction osteogenesis". American Journal of Translational Research. 11 (2): 832–843. PMC 6413255 . PMID 30899383.
- 1 2 Shi, S.; Robey, P. G.; Gronthos, S. (2001). "Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis". Bone. 29 (6): 532–9. doi:10.1016/S8756-3282(01)00612-3. PMID 11728923.
- ↑ Ching, H. S.; Luddin, N.; Rahman, I. A.; Ponnuraj, K. T. (2017). "Expression of Odontogenic and Osteogenic Markers in DPSCS and SHED: A Review". Current Stem Cell Research & Therapy. 12 (1): 71–79. doi:10.2174/1574888X11666160815095733. PMID 27527527.
- 1 2 Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. (2016). "Recent advancements in regenerative dentistry: A review". Materials Science and Engineering: C. 69: 1383–90. doi: 10.1016/j.msec.2016.08.045 . PMID 27612840.
- 1 2 Wu, J.; Li, N.; Fan, Y.; Wang, Y.; Gu, Y.; Li, Z.; Pan, Y.; Romila, G.; Zhou, Z.; Yu, J. (2019). "The Conditioned Medium of Calcined Tooth Powder Promotes the Osteogenic and Odontogenic Differentiation of Human Dental Pulp Stem Cells via MAPK Signaling Pathways". Stem Cells International. 2019: 4793518. doi: 10.1155/2019/4793518 . PMC 6444228 . PMID 31015840.
- ↑ Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K. H. (2005). "Isolation of precursor cells (PCS) from human dental follicle of wisdom teeth". Matrix Biology. 24 (2): 155–65. doi:10.1016/j.matbio.2004.12.004. PMID 15890265.
- ↑ Li, Y.; Yang, Y. Y.; Ren, J. L.; Xu, F.; Chen, F. M.; Li, A. (2017). "Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats". Stem Cell Research & Therapy. 8 (1): 198. doi: 10.1186/s13287-017-0648-5 . PMC 5622448 . PMID 28962585.
- 1 2 3 Yao, S.; Tan, L.; Chen, H.; Huang, X.; Zhao, W.; Wang, Y. (2019). "Potential Research Tool of Stem Cells from Human Exfoliated Deciduous Teeth: Lentiviral Bmi-1 Immortalization with EGFP Marker". Stem Cells International. 2019: 3526409. doi: 10.1155/2019/3526409 . PMC 6431526 . PMID 30984268.
- 1 2 Xiao, L.; Saiki, C.; Okamura, H. (2019). "Oxidative Stress-Tolerant Stem Cells from Human Exfoliated Deciduous Teeth Decrease Hydrogen Peroxide-Induced Damage in Organotypic Brain Slice Cultures from Adult Mice". International Journal of Molecular Sciences. 20 (8): 1858. doi: 10.3390/ijms20081858 . PMC 6514841 . PMID 30991705.
- ↑ Rao, N.; Wang, X.; Zhai, Y.; Li, J.; Xie, J.; Zhao, Y.; Ge, L. (2019). "Stem cells from human exfoliated deciduous teeth ameliorate type II diabetic mellitus in Goto-Kakizaki rats". Diabetology & Metabolic Syndrome. 11: 22. doi: 10.1186/s13098-019-0417-y . PMC 6394089 . PMID 30858895.
- 1 2 3 Dai, Y. Y.; Ni, S. Y.; Ma, K.; Ma, Y. S.; Wang, Z. S.; Zhao, X. L. (2019). "Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro". Stem Cell Research & Therapy. 10 (1): 39. doi: 10.1186/s13287-019-1134-z . PMC 6341645 . PMID 30670101.
- 1 2 3 Yokoyama, T.; Yagi Mendoza, H.; Tanaka, T.; Ii, H.; Takano, R.; Yaegaki, K.; Ishikawa, H. (2019). "Regulation of CCL4-induced liver cirrhosis by hepatically differentiated human dental pulp stem cells" (PDF). Human Cell. 32 (2): 125–140. doi:10.1007/s13577-018-00234-0. PMID 30637566. S2CID 58005147.
- ↑ Mantesso, A.; Sharpe, P. (2009). "Dental stem cells for tooth regeneration and repair". Expert Opinion on Biological Therapy. 9 (9): 1143–54. doi:10.1517/14712590903103795. PMID 19653863. S2CID 10622446.
- ↑ Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L. W.; Robey, P. G.; Shi, S. (2003). "SHED: Stem cells from human exfoliated deciduous teeth". Proceedings of the National Academy of Sciences. 100 (10): 5807–12. Bibcode:2003PNAS..100.5807M. doi: 10.1073/pnas.0937635100 . JSTOR 3147498. PMC 156282 . PMID 12716973.
- ↑ Kerkis, Irina; Kerkis, Alexandre; Dozortsev, Dmitri; Stukart-Parsons, Gaëlle Chopin; Gomes Massironi, Sílvia Maria; Pereira, Lygia V.; Caplan, Arnold I.; Cerruti, Humberto F. (2006). "Isolation and Characterization of a Population of Immature Dental Pulp Stem Cells Expressing OCT-4 and Other Embryonic Stem Cell Markers". Cells Tissues Organs. 184 (3–4): 105–16. doi:10.1159/000099617. PMID 17409736. S2CID 32406588.
- ↑ Graziano, Antonio; d'Aquino, Riccardo; Angelis, Maria Gabriella Cusella-De; De Francesco, Francesco; Giordano, Antonio; Laino, Gregorio; Piattelli, Adriano; Traini, Tonino; De Rosa, Alfredo; Papaccio, Gianpaolo (2008). "Scaffold's surface geometry significantly affects human stem cell bone tissue engineering". Journal of Cellular Physiology. 214 (1): 166–72. doi:10.1002/jcp.21175. PMID 17565721. S2CID 43642451.
- ↑ D'aquino, Riccardo; Papaccio, Gianpaolo; Laino, Gregorio; Graziano, Antonio (2008). "Dental Pulp Stem Cells: A Promising Tool for Bone Regeneration". Stem Cell Reviews. 4 (1): 21–6. doi:10.1007/s12015-008-9013-5. PMID 18300003. S2CID 14784845.
- ↑ Onyekwelu, O; Seppala, M; Zoupa, M; Cobourne, MT (2007). "Tooth development: 2. Regenerating teeth in the laboratory". Dental Update. 34 (1): 20–2, 25–6, 29. doi:10.12968/denu.2007.34.1.20. PMID 17348555.
- ↑ Cordeiro, Mabel M.; Dong, Zhihong; Kaneko, Tomoatsu; Zhang, Zhaocheng; Miyazawa, Marta; Shi, Songtao; Smith, Anthony J.; Nör, Jacques E. (2008). "Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth". Journal of Endodontics. 34 (8): 962–9. doi:10.1016/j.joen.2008.04.009. PMID 18634928.
- ↑ Gandia, Carolina; Armiñan, Ana; García-Verdugo, Jose Manuel; Lledó, Elisa; Ruiz, Amparo; Miñana, M Dolores; Sanchez-Torrijos, Jorge; Payá, Rafael; Mirabet, Vicente; Carbonell-Uberos, Francisco; Llop, Mauro; Montero, Jose Anastasio; Sepúlveda, Pilar (2008). "Human Dental Pulp Stem Cells Improve Left Ventricular Function, Induce Angiogenesis, and Reduce Infarct Size in Rats with Acute Myocardial Infarction". Stem Cells. 26 (3): 638–45. doi: 10.1634/stemcells.2007-0484 . PMID 18079433. S2CID 9594271.
- ↑ Kerkis, Irina; Ambrosio, Carlos E; Kerkis, Alexandre; Martins, Daniele S; Zucconi, Eder; Fonseca, Simone AS; Cabral, Rosa M; Maranduba, Carlos MC; Gaiad, Thais P; Morini, Adriana C; Vieira, Natassia M; Brolio, Marina P; Sant'Anna, Osvaldo A; Miglino, Maria A; Zatz, Mayana (2008). "Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic?". Journal of Translational Medicine. 6: 35. doi: 10.1186/1479-5876-6-35 . PMC 2529267 . PMID 18598348.
- ↑ Nosrat, I; Widenfalk, J; Olson, L; Nosrat, CA (2001). "Dental Pulp Cells Produce Neurotrophic Factors, Interact with Trigeminal Neurons in Vitro, and Rescue Motoneurons after Spinal Cord Injury". Developmental Biology. 238 (1): 120–32. doi: 10.1006/dbio.2001.0400 . PMID 11783998.
- ↑ de Mendonça Costa, André; Bueno, Daniela F.; Martins, Marília T.; Kerkis, Irina; Kerkis, Alexandre; Fanganiello, Roberto D.; Cerruti, Humberto; Alonso, Nivaldo; Passos-Bueno, Maria Rita (2008). "Reconstruction of Large Cranial Defects in Nonimmunosuppressed Experimental Design With Human Dental Pulp Stem Cells". The Journal of Craniofacial Surgery. 19 (1): 204–10. doi:10.1097/scs.0b013e31815c8a54. PMID 18216690. S2CID 23340729.
- ↑ Vishwakarma, Ajaykumar (2014-11-13). Stem Cell Biology and Tissue Engineering in Dental Sciences. Elsevier. ISBN 978-0-12-397157-9.