
A tablet is a pharmaceutical oral dosage form or solid unit dosage form. Tablets may be defined as the solid unit dosage form of medication with suitable excipients. It comprises a mixture of active substances and excipients, usually in powder form, that are pressed or compacted into a solid dose. The main advantages of tablets are that they ensure a consistent dose of medicine that is easy to consume.
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may be used for severe cases of gastroenteritis, especially if the patient is dehydrated.

In pharmacology and toxicology, a route of administration is the way by which a drug, fluid, poison, or other substance is taken into the body.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
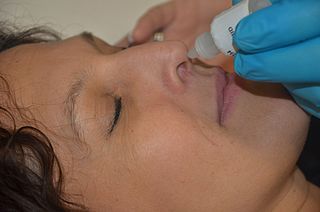
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a tooth. The word topical derives from Greek τοπικόςtopikos, "of a place".
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts, or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerns such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. A comprehensive classification system based on structure-property-application relationships has been proposed for excipients used in parenteral medications.
A polypill is a type of drug combination consisting of a single drug product in pill form and thus combines multiple medications. The prefix "poly" means "multiple", referring to the multiplicity of distinct drugs in a given "pill". In precise usage, a pill is a polypill if it contains at least 4 drugs. An occasional synonym is combopill. A polypill is commonly targets treatment or prevention of chronic conditions.

In the manufacture of pharmaceuticals, encapsulation refers to a range of dosage forms—techniques used to enclose medicines—in a relatively stable shell known as a capsule, allowing them to, for example, be taken orally or be used as suppositories. The two main types of capsules are:
Ampicillin/flucloxacillin (INNs) also known as co-fluampicil (BAN), and sold under the tradename Magnapen, is a combination drug of the two β-lactam antibiotics, ampicillin and flucloxacillin, both in equal amounts, available in a capsule and as a liquid, both taken by mouth, and as a formulation which can be given by injection into muscle or vein.

Oxybutynin, sold as under the brand names Ditropan among others, is a medication used to treat overactive bladder. It works similar to tolterodine, Darifenacin, and Solifenacin. While used for bed wetting in children, evidence to support this use is poor. It is taken by mouth or applied to the skin.

Pill-splitting refers to the practice of splitting a tablet or pill to provide a lower dose of the active ingredient, or to obtain multiple smaller doses, either to reduce cost or because the pills available provide a larger dose than required. Many pills that are suitable for splitting come pre-scored so that they may easily be halved.

"Chasing the dragon" (CTD), or "foily" in Australian English, refers to inhaling the vapor from a heated solution of a powdered psychoactive drug on a sheet of aluminum foil. The moving vapor is chased after with a tube through which the user inhales. The "chasing" occurs as the user gingerly keeps the liquid moving in order to keep it from overheating and burning up too quickly, on a heat conducting material such as aluminium foil.

Drug injection is a method of introducing a drug into the bloodstream via a hollow hypodermic needle, which is pierced through the skin into the body. Intravenous therapy, a form of drug injection, is universally practiced in modernized medical care. As of 2004, there were 13.2 million people worldwide who self-administered injection drugs outside of medical supervision, of which 22% are from developed countries.
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
Modified-release dosage is a mechanism that delivers a drug with a delay after its administration or for a prolonged period of time or to a specific target in the body.

Thin-film drug delivery uses a dissolving film or oral drug strip to administer drugs via absorption in the mouth and/or via the small intestines (enterically). A film is prepared using hydrophilic polymers that rapidly dissolves on the tongue or buccal cavity, delivering the drug to the systemic circulation via dissolution when contact with liquid is made.

Pharmaceutical packaging is the packages and the packaging processes for pharmaceutical preparations. It involves all of the operations from production through drug distribution channels to the end consumer.

Oral administration is a route of administration whereby a substance is taken through the mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications.

The Poison Prevention Packaging Act of 1970 (PPPA); was signed into law by U.S. President Richard Nixon on December 30, 1970. It was enacted by the 91st United States Congress. This law required the use of child-resistant packaging for prescription drugs, over-the-counter (OTC) drugs, household chemicals, and other hazardous materials that could be considered dangerous for children.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.























