
A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.

A medical guideline is a document with the aim of guiding decisions and criteria regarding diagnosis, management, and treatment in specific areas of healthcare. Such documents have been in use for thousands of years during the entire history of medicine. However, in contrast to previous approaches, which were often based on tradition or authority, modern medical guidelines are based on an examination of current evidence within the paradigm of evidence-based medicine. They usually include summarized consensus statements on best practice in healthcare. A healthcare provider is obliged to know the medical guidelines of their profession, and has to decide whether to follow the recommendations of a guideline for an individual treatment.
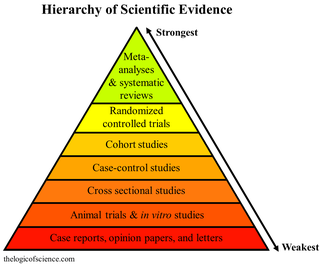
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence. Some case reports also contain a literature review of other reported cases. Case reports are professional narratives that provide feedback on clinical practice guidelines and offer a framework for early signals of effectiveness, adverse events, and cost. They can be shared for medical, scientific, or educational purposes.
In a randomized experiment, allocation concealment hides the sorting of trial participants into treatment groups so that this knowledge cannot be exploited. Adequate allocation concealment serves to prevent study participants from influencing treatment allocations for subjects. Studies with poor allocation concealment are prone to selection bias.

Douglas Graham Altman FMedSci was an English statistician best known for his work on improving the reliability and reporting of medical research and for highly cited papers on statistical methodology. He was professor of statistics in medicine at the University of Oxford, founder and Director of Centre for Statistics in Medicine and Cancer Research UK Medical Statistics Group, and co-founder of the international Equator Network for health research reliability.
Consolidated Standards of Reporting Trials (CONSORT) encompasses various initiatives developed by the CONSORT Group to alleviate the problems arising from inadequate reporting of randomized controlled trials. It is part of the larger EQUATOR Network initiative to enhance the transparency and accuracy of reporting in research.
A clinical prediction rule or clinical probability assessment specifies how to use medical signs, symptoms, and other findings to estimate the probability of a specific disease or clinical outcome.
The STROBE(STrengthening the Reporting of OBservational studies in Epidemiology) Statement is a reporting guideline including a checklist of 22 items that are considered essential for good reporting of observational studies. It was published simultaneously in several leading biomedical journals in October and November 2007 and comprises both the checklist and an explanation and elaboration article which gives examples of good reporting and provides authors with more guidance on good reporting. It is also referred to in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals established by the International Committee of Medical Journal Editors and is endorsed by hundreds of biomedical journals.

John P. A. Ioannidis is a Greek-American physician-scientist, writer and Stanford University professor who has made contributions to evidence-based medicine, epidemiology, and clinical research. Ioannidis studies scientific research itself, meta-research primarily in clinical medicine and the social sciences.

PRISMA is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard superseded the earlier QUOROM standard. It offers the replicability of a systematic literature review. Researchers have to figure out research objectives that answer the research question, states the keywords, a set of exclusion and inclusion criteria. In the review stage, relevant articles were searched, irrelevant ones are removed. Articles are analyzed according to some pre-defined categories.
Alessandro Liberati was an Italian healthcare researcher and clinical epidemiologist, and founder of the Italian Cochrane Centre.
Lesley Ann Stewart is a Scottish academic whose research interests are in the development and application of evidence synthesis methods, particularly systematic reviews and individual participant data meta-analysis. She is head of department for the Centre for Reviews and Dissemination at the University of York and director for the NIHR Evidence Synthesis Programme. She was one of the founders of the Cochrane Collaboration in 1993. Stewart served as president of the Society for Research Synthesis Methodology (2013-2016) and was a founding co-editor in chief of the academic journal Systematic Reviews (2010–2021).
Isabelle Boutron is a professor of epidemiology at the Université Paris Cité and head of the INSERM- METHODS team within the Centre of Research in Epidemiology and Statistics (CRESS). She was originally trained in rheumatology and later switched to a career in epidemiology and public health. She is also deputy director of the French EQUATOR Centre, member of the SPIRIT-CONSORT executive committee, director of Cochrane France and co-convenor of the Bias Methods group of the Cochrane Collaboration.
Metascience is the use of scientific methodology to study science itself. Metascience seeks to increase the quality of scientific research while reducing inefficiency. It is also known as "research on research" and "the science of science", as it uses research methods to study how research is done and find where improvements can be made. Metascience concerns itself with all fields of research and has been described as "a bird's eye view of science". In the words of John Ioannidis, "Science is the best thing that has happened to human beings ... but we can do it better."
Allegiance bias in behavioral sciences is a bias resulted from the investigator's or researcher's allegiance to a specific school of thought. Researchers/investigators have been exposed to many types of branches of psychology or schools of thought. Naturally they adopt a school or branch that fits with their paradigm of thinking. More specifically, allegiance bias is when this leads therapists, researchers, etc. believing that their school of thought or treatment is superior to others. Their superior belief to these certain schools of thought can bias their research in effective treatments trials or investigative situations leading to allegiance bias. Reason being is that they may have devoted their thinking to certain treatments they have seen work in their past experiences. This can lead to errors in interpreting the results of their research. Their “pledge” to stay within their own paradigm of thinking may affect their ability to find more effective treatments to help the patient or situation they are investigating.
The International Prospective Register of Systematic Reviews, better known as PROSPERO, is an open access online database of systematic review protocols on a wide range of topics. While it was initially restricted to medicine, as of 2021, it also accepts protocols in criminology, social care, education and international development, as long as there is a health-related outcome. Researchers can choose to have their reviews prospectively registered with PROSPERO. The database is produced by the Centre for Reviews and Dissemination at the University of York in England, and it is funded by the National Institute for Health Research. Registration of systematic reviews in the database has been supported by PLoS Medicine, BioMed Central, the EQUATOR Network, and BMJ editor-in-chief Fiona Godlee, among others.
Virginia M. Barbour is a professor at Queensland University of Technology in Brisbane, Australia, and serves as the Director of the Australasian Open Access Strategy Group. She is best known for being one of the three founding editors of PLOS Medicine, and her various roles in championing the open access movement.
non-pharmacological intervention (NPI) is any type of healthcare intervention which is not primarily based on medication. Some examples include exercise, sleep improvement, and dietary habits.






