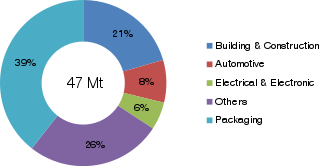
A composite material is a material which is produced from two or more constituent materials. These constituent materials have notably dissimilar chemical or physical properties and are merged to create a material with properties unlike the individual elements. Within the finished structure, the individual elements remain separate and distinct, distinguishing composites from mixtures and solid solutions.
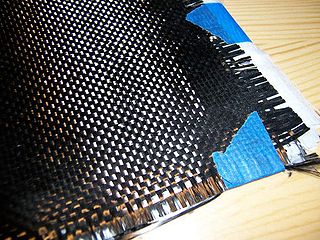
Carbon fibers or carbon fibres are fibers about 5 to 10 micrometers (0.00020–0.00039 in) in diameter and composed mostly of carbon atoms. Carbon fibers have several advantages: high stiffness, high tensile strength, high strength to weight ratio, high chemical resistance, high-temperature tolerance, and low thermal expansion. These properties have made carbon fiber very popular in aerospace, civil engineering, military, motorsports, and other competition sports. However, they are relatively expensive compared to similar fibers, such as glass fiber, basalt fibers, or plastic fibers.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Plastic welding is welding for semi-finished plastic materials, and is described in ISO 472 as a process of uniting softened surfaces of materials, generally with the aid of heat. Welding of thermoplastics is accomplished in three sequential stages, namely surface preparation, application of heat and pressure, and cooling. Numerous welding methods have been developed for the joining of semi-finished plastic materials. Based on the mechanism of heat generation at the welding interface, welding methods for thermoplastics can be classified as external and internal heating methods, as shown in Fig 1.

Wollastonite is a calcium inosilicate mineral (CaSiO3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or dolomite is subjected to high temperature and pressure, which sometimes occurs in the presence of silica-bearing fluids as in skarns or in contact with metamorphic rocks. Associated minerals include garnets, vesuvianite, diopside, tremolite, epidote, Plagioclase feldspar, pyroxene and calcite. It is named after the English chemist and mineralogist William Hyde Wollaston (1766–1828).
Fibre-reinforced plastic is a composite material made of a polymer matrix reinforced with fibres. The fibres are usually glass, carbon, aramid, or basalt. Rarely, other fibres such as paper, wood, boron, or asbestos have been used. The polymer is usually an epoxy, vinyl ester, or polyester thermosetting plastic, though phenol formaldehyde resins are still in use.
Zytel is a trademark owned by Celanese and used for a number of different high strength, abrasion and impact resistant thermoplastic polyamide formulations of the family more commonly known as nylon. The Zytel product line is based mostly on nylon 66, but also includes grades based on nylon 6 as a matrix, long chain nylons such as nylon 610, and copolymers including a transparent resin called Zytel 330. Resins based on polyphthalamides are branded 'Zytel HTN'. The Zytel product range takes advantage of the fact that nylons are one of the most compatible polymers with modifiers and so offers grades with varying degrees of fiberglass, from 13% to 60%,, rubber toughened resins, flame retarded grades. Nylon resins with mineral reinforcement are branded 'Minlon'.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.
Rubber toughening is a process in which rubber nanoparticles are interspersed within a polymer matrix to increase the mechanical robustness, or toughness, of the material. By "toughening" a polymer it is meant that the ability of the polymeric substance to absorb energy and plastically deform without fracture is increased. Considering the significant advantages in mechanical properties that rubber toughening offers, most major thermoplastics are available in rubber-toughened versions; for many engineering applications, material toughness is a deciding factor in final material selection.

Solid is one of the four fundamental states of matter, thus along with liquid, gas, and to add to it not many people know this one but it is plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
Carbon fiber-reinforced polymers, carbon-fibre-reinforced polymers, carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic, also known as carbon fiber, carbon composite, or just carbon, are extremely strong and light fiber-reinforced plastics that contain carbon fibers. CFRPs can be expensive to produce, but are commonly used wherever high strength-to-weight ratio and stiffness (rigidity) are required, such as aerospace, superstructures of ships, automotive, civil engineering, sports equipment, and an increasing number of consumer and technical applications.
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.

Paper chemicals designate a group of chemicals that are used for paper manufacturing, or modify the properties of paper. These chemicals can be used to alter the paper in many ways, including changing its color and brightness, or by increasing its strength and resistance to water. The chemicals can be defined on basis of their usage in the process.
A masterbatch is a concentrated mixture of additives in a carrier matrix which is used to conveniently add these additives to the final plastic product. The additives may be used for coloring or for imparting other properties. The alternative to using masterbatches is compounding the plastic from the raw undiluted additive.
Novel polymeric alloy (NPA) is a polymeric alloy composed of polyolefin and thermoplastic engineering polymer with enhanced engineering properties. NPA was developed for use in geosynthetics. One of the first commercial NPA applications was in the manufacturer of polymeric strips used to form Neoloy® cellular confinement systems (geocells).

Acrylonitrile styrene acrylate (ASA), also called acrylic styrene acrylonitrile, is an amorphous thermoplastic developed as an alternative to acrylonitrile butadiene styrene (ABS), that has improved weather resistance. It is an acrylate rubber-modified styrene acrylonitrile copolymer. It is used for general prototyping in 3D printing, where its UV resistance and mechanical properties make it an excellent material for use in fused filament fabrication printers, particularly for outdoor applications. ABS is also widely used in the automotive industry.
Thermoplastics containing short fiber reinforcements were first introduced commercially in the 1960s. The most common type of fibers used in short fiber thermoplastics are glass fiber and carbon fiber . Adding short fibers to thermoplastic resins improves the composite performance for lightweight applications. In addition, short fiber thermoplastic composites are easier and cheaper to produce than continuous fiber reinforced composites. This compromise between cost and performance allows short fiber reinforced thermoplastics to be used in myriad applications.