Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection. In those with both HIV/AIDS and HBV antiretroviral medication should also be used. Entecavir is taken by mouth as a tablet or solution.

Taribavirin is an antiviral drug in Phase III human trials, but not yet approved for pharmaceutical use. It is a prodrug of ribavirin, active against a number of DNA and RNA viruses. Taribavirin has better liver-targeting than ribavirin, and has a shorter life in the body due to less penetration and storage in red blood cells. It is expected eventually to be the drug of choice for viral hepatitis syndromes in which ribavirin is active. These include hepatitis C and perhaps also hepatitis B and yellow fever.

Alisporivir (INN), or Debio 025, DEB025, is a cyclophilin inhibitor. Its structure is reminiscent of, and synthesized from ciclosporin.
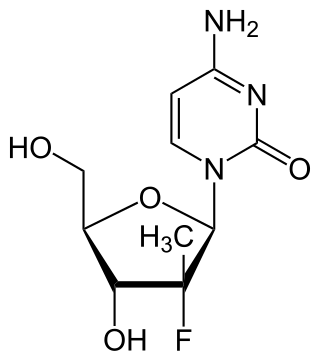
PSI-6130 is an experimental treatment for hepatitis C. PSI-6130 is a member of a class of antiviral drugs known as nucleoside polymerase inhibitors that was created by chemist Jeremy L. Clark. Specifically, PSI-6130 inhibits the hepatitis C virus RNA dependant RNA polymerase called NS5B.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. It was invented by Professor Chris McGuigan in the early 1990s. They form a critical part of the anti-viral drugs: sofosbuvir, tenofovir alafenamide, and remdesivir.
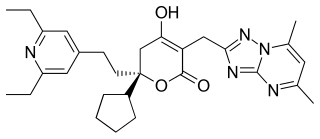
Filibuvir was a non-nucleoside orally available NS5B inhibitor developed by Pfizer for the treatment of hepatitis C. It binds to the non-catalytic Thumb II allosteric pocket of NS5B viral polymerase and causes a decrease in viral RNA synthesis. It is a potent and selective inhibitor, with a mean IC50 of 0.019 μM against genotype 1 polymerases. Several filibuvir-resistant mutations have been identified, M423 being the most common that occurred after filibuvir monotherapy. It was intended to be taken twice-daily.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.

Glecaprevir (INN,) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals. It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance in vitro.
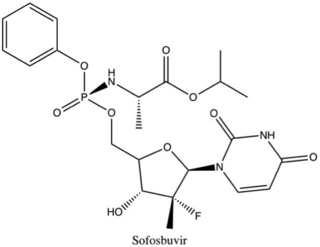
Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.

Lobucavir is an antiviral drug that shows broad-spectrum activity against herpesviruses, hepatitis B and other hepadnaviruses, HIV/AIDS and cytomegalovirus. It initially demonstrated positive results in human clinical trials against hepatitis B with minimal adverse effects but was discontinued from further development following the discovery of increased risk of cancer associated with long-term use in mice. Although this carcinogenic risk is present in other antiviral drugs, such as zidovudine and ganciclovir that have been approved for clinical use, development was halted by Bristol-Myers Squibb, its manufacturer.
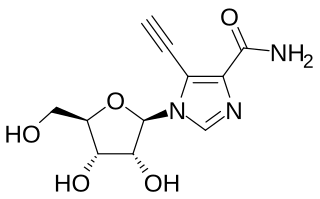
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.

North-Methanocarbathymidine (N-MCT) is an antiviral drug which is an analogue of thymidine, and shows activity against herpesviruses, orthopoxviruses and HIV, though it has not been introduced into clinical use.
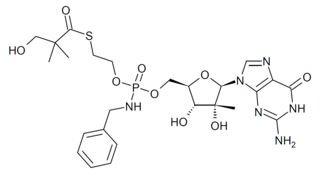
IDX-184 is an antiviral drug which was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor. While it showed reasonable effectiveness in early clinical trials it did not progress past Phase IIb. However research using this drug has continued as it shows potentially useful activity against other emerging viral diseases such as Zika virus, and coronaviruses including MERS, and SARS-CoV-2.

Valopicitabine (NM-283) is an antiviral drug which was developed as a treatment for hepatitis C, though only progressed as far as Phase III clinical trials. It acts as a RNA-dependent RNA polymerase inhibitor. It is a prodrug which is converted inside the body to the active form, 2'-C-methylcytidine triphosphate.

Sangivamycin is a natural product originally isolated from Streptomyces rimosus, which is a nucleoside analogue. It acts as an inhibitor of protein kinase C. It has antibiotic, antiviral and anti-cancer properties and has been investigated for various medical applications, though never approved for clinical use itself. However, a number of related derivatives continue to be researched.
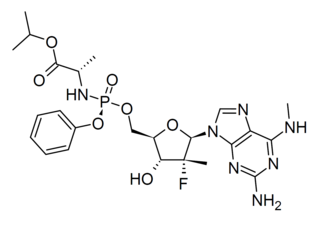
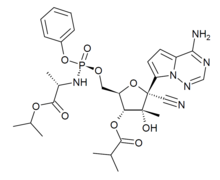
Bemnifosbuvir is an antiviral drug invented by Atea Pharmaceuticals and licensed to Roche for clinical development, a novel nucleotide analog prodrug originally developed for the treatment of hepatitis C. AT-527 is the orally bioavailable hemisulfate salt of AT-511, which is metabolised in several steps to the active nucleotide triphosphate AT-9010, acting as an RNA polymerase inhibitor and thereby interfering with viral replication. AT-527 has been researched for the treatment of coronavirus diseases such as that produced by SARS-CoV-2. It showed good results in early clinical trials but had inconsistent results at later stages, so the planned Phase 3 trials are being redesigned and results are not expected until late 2022.