Research methods
Antisense oligonucleotides
Antisense oligonucleotides were discovered in 1978 by Paul Zamecnik and Mary Stephenson. [5] Oligonucleotides, which are short nucleic acid fragments, bind to complementary target mRNA molecules when added to the cell. [5] [6] These molecules can be composed of single-stranded DNA or RNA and are generally 13–25 nucleotides long. [6] [7] The antisense oligonucleotides can affect gene expression in two ways: by using an RNase H-dependent mechanism or by using a steric blocking mechanism. [6] [7] RNase H-dependent oligonucleotides cause the target mRNA molecules to be degraded, while steric-blocker oligonucleotides prevent translation of the mRNA molecule. [6] [7] The majority of antisense drugs function through the RNase H-dependent mechanism, in which RNase H hydrolyzes the RNA strand of the DNA/RNA heteroduplex. [6] [7] expression. [6]
Ribozymes

Ribozymes are catalytic RNA molecules used to inhibit gene expression. These molecules work by cleaving mRNA molecules, essentially silencing the genes that produced them. Sidney Altman and Thomas Cech first discovered catalytic RNA molecules, RNase P and group II intron ribozymes, in 1989 and won the Nobel Prize for their discovery. [8] [9] Several types of ribozyme motifs exist, including hammerhead, hairpin, hepatitis delta virus, group I, group II, and RNase P ribozymes. Hammerhead, hairpin, and hepatitis delta virus (HDV) ribozyme motifs are generally found in viruses or viroid RNAs. [8] These motifs are able to self-cleave a specific phosphodiester bond on an mRNA molecule. [8] Lower eukaryotes and a few bacteria contain group I and group II ribozymes. [8] These motifs can self-splice by cleaving and joining phosphodiester bonds. [8] The last ribozyme motif, the RNase P ribozyme, is found in Escherichia coli and is known for its ability to cleave the phosphodiester bonds of several tRNA precursors when joined to a protein cofactor. [8]
The general catalytic mechanism used by ribozymes is similar to the mechanism used by protein ribonucleases. [10] These catalytic RNA molecules bind to a specific site and attack the neighboring phosphate in the RNA backbone with their 2' oxygen, which acts as a nucleophile, resulting in the formation of cleaved products with a 2'3'-cyclic phosphate and a 5' hydroxyl terminal end. [10] This catalytic mechanism has been increasingly used by scientists to perform sequence-specific cleavage of target mRNA molecules. In addition, attempts are being made to use ribozymes to produce gene silencing therapeutics, which would silence genes that are responsible for causing diseases. [11]
RNA interference

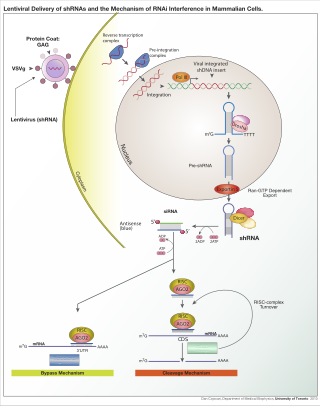
RNA interference (RNAi) is a natural process used by cells to regulate gene expression. It was discovered in 1998 by Andrew Fire and Craig Mello, who won the Nobel Prize for their discovery in 2006. [12] The process to silence genes first begins with the entrance of a double-stranded RNA (dsRNA) molecule into the cell, which triggers the RNAi pathway. [12] The double-stranded molecule is then cut into small double-stranded fragments by an enzyme called Dicer. [12] These small fragments, which include small interfering RNAs (siRNA) and microRNA (miRNA), are approximately 21–23 nucleotides in length. [12] [13] The fragments integrate into a multi-subunit protein called the RNA-induced silencing complex, which contains Argonaute proteins that are essential components of the RNAi pathway. [12] [13] One strand of the molecule, called the "guide" strand, binds to RISC, while the other strand, known as the "passenger" strand is degraded. [12] [13] The guide or antisense strand of the fragment that remains bound to RISC directs the sequence-specific silencing of the target mRNA molecule. [13] The genes can be silenced by siRNA molecules that cause the endonucleatic cleavage of the target mRNA molecules or by miRNA molecules that suppress translation of the mRNA molecule. [13] With the cleavage or translational repression of the mRNA molecules, the genes that form them are rendered essentially inactive. [12] RNAi is thought to have evolved as a cellular defense mechanism against invaders, such as RNA viruses, or to combat the proliferation of transposons within a cell's DNA. [12] Both RNA viruses and transposons can exist as double-stranded RNA and lead to the activation of RNAi. [12] Currently, siRNAs are being widely used to suppress specific gene expression and to assess the function of genes. Companies utilizing this approach include Alnylam, Sanofi, [14] Arrowhead, Discerna, [15] and Persomics, [16] among others.
Three prime untranslated regions and microRNAs
The three prime untranslated regions (3'UTRs) of messenger RNAs (mRNAs) often contain regulatory sequences that post-transcriptionally cause gene silencing. Such 3'-UTRs often contain both binding sites for microRNAs (miRNAs) as well as for regulatory proteins. By binding to specific sites within the 3'-UTR, a large number of specific miRNAs decrease gene expression of their particular target mRNAs by either inhibiting translation or directly causing degradation of the transcript, using a mechanism similar to RNA interference (see MicroRNA). The 3'-UTR also may have silencer regions that bind repressor proteins that inhibit the expression of an mRNA.[ citation needed ]
The 3'-UTR often contains microRNA response elements (MREs). MREs are sequences to which miRNAs bind and cause gene silencing. These are prevalent motifs within 3'-UTRs. Among all regulatory motifs within the 3'-UTRs (e.g. including silencer regions), MREs make up about half of the motifs.[ citation needed ]
As of 2014, the miRBase web site, [17] an archive of miRNA sequences and annotations, listed 28,645 entries in 233 biologic species. Of these, 1,881 miRNAs were in annotated human miRNA loci. miRNAs were predicted to each have an average of about four hundred target mRNAs (causing gene silencing of several hundred genes). [18] Freidman et al. [18] estimate that >45,000 miRNA target sites within human mRNA 3'UTRs are conserved above background levels, and >60% of human protein-coding genes have been under selective pressure to maintain pairing to miRNAs.[ citation needed ]
Direct experiments show that a single miRNA can reduce the stability of hundreds of unique mRNAs. [19] Other experiments show that a single miRNA may repress the production of hundreds of proteins, but that this repression often is relatively mild (less than 2-fold). [20] [21]
The effects of miRNA dysregulation of gene expression seem to be important in cancer. [22] For instance, in gastrointestinal cancers, nine miRNAs have been identified as epigenetically altered and effective in down regulating DNA repair enzymes. [23]
The effects of miRNA dysregulation of gene expression also seem to be important in neuropsychiatric disorders, such as schizophrenia, bipolar disorder, major depression, Parkinson's disease, Alzheimer's disease and autism spectrum disorders. [24] [25] [26]