Prostate cancer is the uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Early prostate cancer causes no symptoms. Abnormal growth of prostate tissue is usually detected through screening tests, typically blood tests that check for prostate-specific antigen (PSA) levels. Those with high levels of PSA in their blood are at increased risk for developing prostate cancer. Diagnosis requires a biopsy of the prostate. If cancer is present, the pathologist assigns a Gleason score, and a higher score represents a more dangerous tumor. Medical imaging is performed to look for cancer that has spread outside the prostate. Based on the Gleason score, PSA levels, and imaging results, a cancer case is assigned a stage 1 to 4. Higher stage signifies a more advanced, more dangerous disease.

Thyroid neoplasm is a neoplasm or tumor of the thyroid. It can be a benign tumor such as thyroid adenoma, or it can be a malignant neoplasm, such as papillary, follicular, medullary or anaplastic thyroid cancer. Most patients are 25 to 65 years of age when first diagnosed; women are more affected than men. The estimated number of new cases of thyroid cancer in the United States in 2023 is 43,720 compared to only 2,120 deaths. Of all thyroid nodules discovered, only about 5 percent are cancerous, and under 3 percent of those result in fatalities.

Adenocarcinoma is a type of cancerous tumor that can occur in several parts of the body. It is defined as neoplasia of epithelial tissue that has glandular origin, glandular characteristics, or both. Adenocarcinomas are part of the larger grouping of carcinomas, but are also sometimes called by more precise terms omitting the word, where these exist. Thus invasive ductal carcinoma, the most common form of breast cancer, is adenocarcinoma but does not use the term in its name—however, esophageal adenocarcinoma does to distinguish it from the other common type of esophageal cancer, esophageal squamous cell carcinoma. Several of the most common forms of cancer are adenocarcinomas, and the various sorts of adenocarcinoma vary greatly in all their aspects, so that few useful generalizations can be made about them.

Carcinoma is a malignancy that develops from epithelial cells. Specifically, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that arises from cells originating in the endodermal, mesodermal or ectodermal germ layer during embryogenesis.

An adenoma is a benign tumor of epithelial tissue with glandular origin, glandular characteristics, or both. Adenomas can grow from many glandular organs, including the adrenal glands, pituitary gland, thyroid, prostate, and others. Some adenomas grow from epithelial tissue in nonglandular areas but express glandular tissue structure. Although adenomas are benign, they should be treated as pre-cancerous. Over time adenomas may transform to become malignant, at which point they are called adenocarcinomas. Most adenomas do not transform. However, even though benign, they have the potential to cause serious health complications by compressing other structures and by producing large amounts of hormones in an unregulated, non-feedback-dependent manner. Some adenomas are too small to be seen macroscopically but can still cause clinical symptoms.

Pseudomyxoma peritonei (PMP) is a clinical condition caused by cancerous cells that produce abundant mucin or gelatinous ascites. The tumors cause fibrosis of tissues and impede digestion or organ function, and if left untreated, the tumors and mucin they produce will fill the abdominal cavity. This will result in compression of organs and will destroy the function of the colon, small intestine, stomach, or other organs. Prognosis with treatment in many cases is optimistic, but the disease is lethal if untreated, with death occurring via cachexia, bowel obstruction, or other types of complications.

Invasive carcinoma of no special type, invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), infiltrating ductal carcinoma (IDC) or invasive ductal carcinoma, not otherwise specified (NOS) is a disease. For international audiences this article will use "invasive carcinoma NST" because it is the preferred term of the World Health Organization (WHO).
Prostate cancer staging is the process by which physicians categorize the risk of cancer having spread beyond the prostate, or equivalently, the probability of being cured with local therapies such as surgery or radiation. Once patients are placed in prognostic categories, this information can contribute to the selection of an optimal approach to treatment. Prostate cancer stage can be assessed by either clinical or pathological staging methods. Clinical staging usually occurs before the first treatment and tumour presence is determined through imaging and rectal examination, while pathological staging is done after treatment once a biopsy is performed or the prostate is removed by looking at the cell types within the sample.

In pathology, grading is a measure of the cell appearance in tumors and other neoplasms. Some pathology grading systems apply only to malignant neoplasms (cancer); others apply also to benign neoplasms. The neoplastic grading is a measure of cell anaplasia in the sampled tumor and is based on the resemblance of the tumor to the tissue of origin. Grading in cancer is distinguished from staging, which is a measure of the extent to which the cancer has spread.

Transitional cell carcinoma, also called urothelial carcinoma, is a type of cancer that typically occurs in the urinary system. It is the most common type of bladder cancer and cancer of the ureter, urethra, and urachus. Symptoms of urothelial carcinoma in the bladder include hematuria. Diagnosis includes urine analysis and imaging of the urinary tract (cystoscopy). Transitional cell carcinomas arise from the transitional epithelium, a tissue lining the inner surface of these hollow organs. When the term "urothelial" is used, it specifically refers to a carcinoma of the urothelium, meaning a transitional cell carcinomas of the urinary system.

Salivary gland tumours, also known as mucous gland adenomas or neoplasms, are tumours that form in the tissues of salivary glands. The salivary glands are classified as major or minor. The major salivary glands consist of the parotid, submandibular, and sublingual glands. The minor salivary glands consist of 800 to 1000 small mucus-secreting glands located throughout the lining of the oral cavity. Patients with these types of tumours may be asymptomatic.
Comedocarcinoma is a kind of breast cancer that demonstrates comedonecrosis, which is the central necrosis of cancer cells within involved ducts. Comedocarcinomas are usually non-infiltrating and intraductal tumors, characterized as a comedo-type, high-grade ductal carcinoma in situ (DCIS). However, there have been accounts of comedocarcinoma which has then diversified into other cell types and developed into infiltrating (invasive) ductal carcinoma. Recurrence and survival rates differ for invasive breast cancer which has originated as comedocarcinoma compared with other types of cancer cells.

High-grade prostatic intraepithelial neoplasia (HGPIN) is an abnormality of prostatic glands and believed to precede the development of prostate adenocarcinoma.
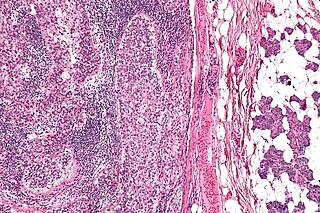
Sebaceous carcinoma, also known as sebaceous gland carcinoma (SGc), sebaceous cell carcinoma, and meibomian gland carcinoma is an uncommon malignant cutaneous tumor. Most are typically about 1.4 cm at presentation. SGc originates from sebaceous glands in the skin and, therefore, may originate anywhere in the body where these glands are found. SGc can be divided into 2 types: periocular and extraocular. The periocular region is rich in sebaceous glands making it a common site of origin. The cause of these lesions in the vast majority of cases is unknown. Occasional cases may be associated with Muir-Torre syndrome. SGc accounts for approximately 0.7% of all skin cancers, and the incidence of SGc is highest in Caucasian, Asian, and Indian populations. Due to the rarity of this tumor and variability in clinical and histological presentation, SGc is often misdiagnosed as an inflammatory condition or a more common neoplasm. SGc is commonly treated with wide local excision or Mohs micrographic surgery, and the relative survival rates at 5 and 10 years are 92.72 and 86.98%, respectively.
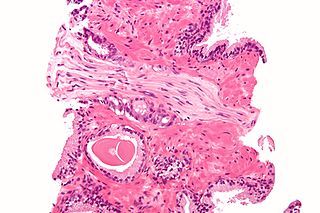
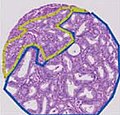
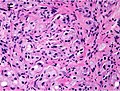
In pathology, perineural invasion, abbreviated PNI, refers to the invasion of cancer to the space surrounding a nerve. It is common in head and neck cancer, prostate cancer and colorectal cancer.
Acinar adenocarcinoma is a histological subtype of gland-forming cancer that is diagnosed when cuboidal and/or columnar shaped malignant cells in the neoplastic tissue form acini and tubules. It is a common form of cancer occurring in the lung and prostate gland.
A histopathologic diagnosis of prostate cancer is the discernment of whether there is a cancer in the prostate, as well as specifying any subdiagnosis of prostate cancer if possible. The histopathologic subdiagnosis of prostate cancer has implications for the possibility and methodology of any subsequent Gleason scoring. The most common histopathological subdiagnosis of prostate cancer is acinar adenocarcinoma, constituting 93% of prostate cancers. The most common form of acinar adenocarcinoma, in turn, is "adenocarcinoma, not otherwise specified", also termed conventional, or usual acinar adenocarcinoma.

The histopathology of colorectal cancer of the adenocarcinoma type involves analysis of tissue taken from a biopsy or surgery. A pathology report contains a description of the microscopical characteristics of the tumor tissue, including both tumor cells and how the tumor invades into healthy tissues and finally if the tumor appears to be completely removed. The most common form of colon cancer is adenocarcinoma, constituting between 95% and 98% of all cases of colorectal cancer. Other, rarer types include lymphoma, adenosquamous and squamous cell carcinoma. Some subtypes have been found to be more aggressive.
Papillary carcinomas of the breast (PCB), also termed malignant papillary carcinomas of the breast, are rare forms of the breast cancers. The World Health Organization (2019) classified papillary neoplasms of the breast into 5 types: intraductal papilloma, papillary ductal carcinoma in situ (PDCIS), encapsulated papillary carcinoma (EPC), solid-papillary carcinoma (SPC), and invasive papillary carcinoma (IPC). The latter four carcinomas are considered here; intraductal papilloma is a benign neoplasm. The World Health Organization regarded solid papillary carcinoma as having two subtypes: in situ and invasive SPC.

Invasive cribriform carcinoma of the breast (ICCB), also termed invasive cribriform carcinoma, is a rare type of breast cancer that accounts for 0.3% to 0.6% of all carcinomas in the breast. It originates in a lactiferous duct as opposed to the lobules that form the alveoli in the breasts' mammary glands. ICCB was first described by Dixon and colleagues in 1983 as a tumor that on microscopic histopathological inspection had a cribriform pattern, i.e. a tissue pattern consisting of numerous "Swiss cheese"-like open spaces and/or sieve-like small holes. The latest edition (2019) of the World Health Organization (2019) termed these lesions invasive cribriform carcinomas indicating that by definition they must have a component that invades out of their ducts of origin into adjacent tissues. In situ ductal cancers that have a cribriform histopathology are regarded as belonging to the group of ductal carcinoma in situ tumors.