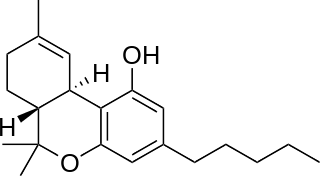
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the Delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. THC is a terpenoid found in cannabis and, like many pharmacologically active phytochemicals, it is assumed to be involved in the plant's evolutionary adaptation against insect predation, ultraviolet light, and environmental stress. THC was first discovered and isolated by Israeli chemist Raphael Mechoulam in Israel in 1964. It was found that, when smoked, THC is absorbed into the bloodstream and travels to the brain, attaching itself to endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia. These are the parts of the brain responsible for thinking, memory, pleasure, coordination and movement.

Liquorice or licorice is the common name of Glycyrrhiza glabra, a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring is extracted.

Oxiracetam is a nootropic drug of the racetam family and a very mild stimulant. Several studies suggest that the substance is safe even when high doses are consumed for a long period of time. However, the mechanism of action of the racetam drug family is still a matter of research. Oxiracetam is not approved by Food and Drug Administration for any medical use in the United States.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.
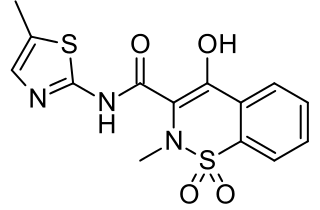
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory medication (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Liquorice or licorice is a confection usually flavoured and coloured black with the extract of the roots of the liquorice plant Glycyrrhiza glabra.

Enoxolone is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. It is effective in the treatment of peptic ulcer and also has expectorant (antitussive) properties. It has some additional pharmacological properties with possible antiviral, antifungal, antiprotozoal, and antibacterial activities.

Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besides hypertension, it is also used in diabetic kidney disease, heart failure, and left ventricular enlargement. It comes as a tablet that is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.
Secondary hypertension is a type of hypertension which by definition is caused by an identifiable underlying primary cause. It is much less common than the other type, called essential hypertension, affecting only 5-10% of hypertensive patients. It has many different causes including endocrine diseases, kidney diseases, and tumors. It also can be a side effect of many medications.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant.

Glycyrrhiza uralensis, also known as Chinese liquorice, is a flowering plant native to Asia. It is used as a sweetener and in traditional Chinese medicine.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a derivative of coumarin. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.

Erdosteine is a molecule with mucolytic activity. Structurally it is a thiol derivative characterized by the presence of two thiol groups. These two functional sulfhydryl groups contained in the molecule are released following first-pass metabolism with the conversion of erdosteine into its pharmacologically active metabolite Met-I.

11-Nor-9-carboxy-Δ9-tetrahydrocannabinol, often referred to as 11-nor-9-carboxy-THC or THC-11-oic acid, is the main secondary metabolite of tetrahydrocannabinol (THC) which is formed in the body after cannabis is consumed.
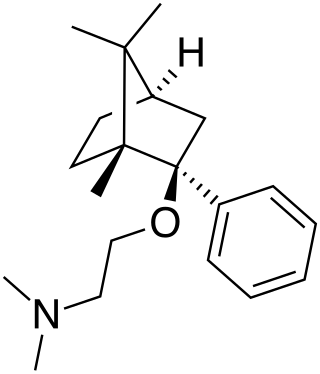
Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.

Edoxaban, sold under the brand name Lixiana among others, is an anticoagulant medication and a direct factor Xa inhibitor. It is taken by mouth.
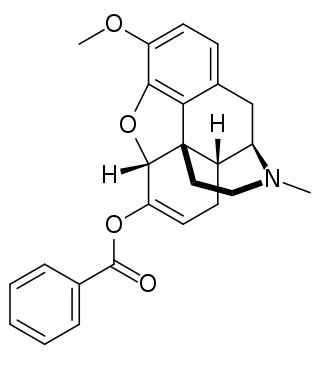
Benzhydrocodone (INN) is an opioid prodrug of the morphinan class. Its chemical structure consists of hydrocodone coupled with benzoic acid. Benzhydrocodone itself is inactive and acts as a prodrug to hydrocodone upon cleavage of the benzoate portion of the molecule.

Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant Mitragyna speciosa, commonly known as kratom. The total alkaloid concentration in dried leaves ranges from 0.5 to 1.5%. In Thai varieties, mitragynine is the most abundant component while 7-hydroxymitragynine is a minor constituent. In Malaysian kratom varieties, mitragynine is present at lower concentration. Such preparations are orally consumed and typically involve dried kratom leaves which are brewed into tea or ground and placed into capsules. Mitragynine consumption for medicinal and recreation purposes dates back centuries, although early use was primarily limited to Southeast Asian countries such as Indonesia and Thailand where the plant grows indigenously. Recently, mitragynine use has spread throughout Europe and the Americas as both a recreational and medicinal drug. While research into the effects of kratom have begun to emerge, investigations on the active compound mitragynine are less common.




















