Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. As a result, kinase produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.
Gluconeogenesis (GNG) is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.

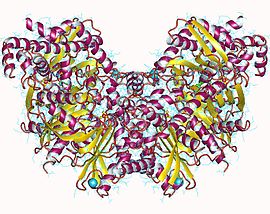
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

Phosphoglucomutase is an enzyme that transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward direction or the 6 to the 1 position in the reverse direction.
Chemical specificity is the ability of binding site of a macromolecule to bind specific ligands. The fewer ligands a protein can bind, the greater its specificity.

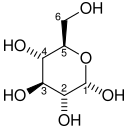
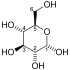
Glucose 6-phosphate is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.

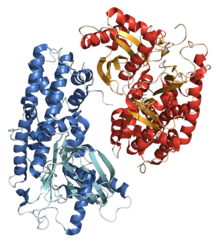
Glucokinase is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia.

The Entner–Doudoroff pathway is a metabolic pathway that is most notable in Gram-negative bacteria, certain Gram-positive bacteria and archaea. Glucose is the substrate in the ED pathway and through a series of enzyme assisted chemical reactions it is catabolized into pyruvate. Entner and Doudoroff (1952) and MacGee and Doudoroff (1954) first reported the ED pathway in the bacterium Pseudomonas saccharophila. While originally thought to be just an alternative to glycolysis (EMP) and the pentose phosphate pathway (PPP), some studies now suggest that the original role of the EMP may have originally been about anabolism and repurposed over time to catabolism, meaning the ED pathway may be the older pathway. Recent studies have also shown the prevalence of the ED pathway may be more widespread than first predicted with evidence supporting the presence of the pathway in cyanobacteria, ferns, algae, mosses, and plants. Specifically, there is direct evidence that Hordeum vulgare uses the Entner–Doudoroff pathway.

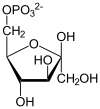
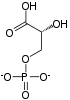
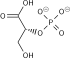
Fructose 1,6-bisphosphate, known in older publications as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6. The β-D-form of this compound is common in cells. Upon entering the cell, most glucose and fructose is converted to fructose 1,6-bisphosphate.

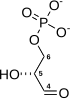
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-P2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-P2 itself is synthesized and broken down in either direction by the integrated bifunctional enzyme phosphofructokinase 2 (PFK-2/FBPase-2), which also contains a phosphatase domain and is also known as fructose-2,6-bisphosphatase. Whether the kinase and phosphatase domains of PFK-2/FBPase-2 are active or inactive depends on the phosphorylation state of the enzyme.

Enzyme activators are molecules that bind to enzymes and increase their activity. They are the opposite of enzyme inhibitors. These molecules are often involved in the allosteric regulation of enzymes in the control of metabolism. An example of an enzyme activator working in this way is fructose 2,6-bisphosphate, which activates phosphofructokinase 1 and increases the rate of glycolysis in response to the hormone glucagon. In some cases, when a substrate binds to one catalytic subunit of an enzyme, this can trigger an increase in the substrate affinity as well as catalytic activity in the enzyme's other subunits, and thus the substrate acts as an activator.

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Unlike glucose, which is directly metabolized widely in the body, fructose is mostly metabolized in the liver in humans, where it is directed toward replenishment of liver glycogen and triglyceride synthesis. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.

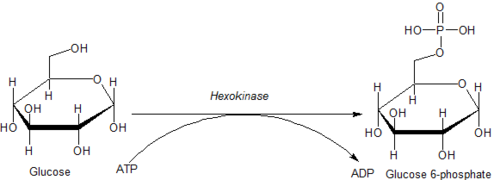
Hexokinase 2 also known as HK2 is an enzyme which in humans is encoded by the HK2 gene on chromosome 2. Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes hexokinase 2, the predominant form found in skeletal muscle. It localizes to the outer membrane of mitochondria. Expression of this gene is insulin-responsive, and studies in rat suggest that it is involved in the increased rate of glycolysis seen in rapidly growing cancer cells. [provided by RefSeq, Apr 2009]

Hexokinase 3 also known as HK3 is an enzyme which in humans is encoded by the HK3 gene on chromosome 5. Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes hexokinase 3. Similar to hexokinases 1 and 2, this allosteric enzyme is inhibited by its product glucose-6-phosphate. [provided by RefSeq, Apr 2009]

The TP53-inducible glycolysis and apoptosis regulator (TIGAR) also known as fructose-2,6-bisphosphatase TIGAR is an enzyme that in humans is encoded by the C12orf5 gene.