Etymology and usage
The word idiotype comes from two Greek roots, idio meaning 'private, distinctive, peculiar' and typos meaning 'mark.' Thus, idiotype describes the distinctive sequence and region that makes any immunoglobulin/TCR unique from others of the same type which is its variable region.
The term "idiotype" is sometimes used to describe the collection of multiple idiotopes, and therefore overall antigen binding capacity, possessed by an antibody.
The word "idiotype" became influential in immunology when Niels Jerne formulated his immune network theory. Jerne was awarded the Nobel Prize in Physiology or Medicine in 1984 largely for being the father of immune network theory. He defined idiotype as the set of epitopes on the V region of an antibody molecule, where epitope means an antigenic determinant. He also defined the "paratope" to be that part of an antibody variable region that binds to an antigen. The best developed version of immune network theory is called the symmetrical network theory, in which the distinction between idiotype and paratope plays no role.

In immunology, an antigen (Ag) is a molecule or molecular structure that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. The term antigen originally referred to a substance that is an antibody generator. Antigens can be proteins, peptides, polysaccharides, lipids, nucleic acids, or other biomolecules.

An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.
An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. The epitope is the specific piece of the antigen to which an antibody binds. The part of an antibody that binds to the epitope is called a paratope. Although epitopes are usually non-self proteins, sequences derived from the host that can be recognized are also epitopes.

The adaptive immune system, also referred as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.
Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens, or from phagocytosed pathogens ; subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigen before they are stably expressed on a cell surface. MHC I antigen presentation typically involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.

Clonal selection theory is a scientific theory in immunology that explains the functions of cells of the immune system (lymphocytes) in response to specific antigens invading the body. The concept was introduced by Australian doctor Frank Macfarlane Burnet in 1957, in an attempt to explain the great diversity of antibodies formed during initiation of the immune response. The theory has become the widely accepted model for how the human immune system responds to infection and how certain types of B and T lymphocytes are selected for destruction of specific antigens.
V(D)J recombination is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system.
An idiotope is the unique set of antigenic determinants (epitopes) of the variable portion of an antibody. In some cases it can be the actual antigen-binding site, and in some cases it may comprise variable region sequences outside of the antigen-binding site on the antibody itself. Thus each antibody would have multiple idiotopes; and the set of these individual idiotopes is termed the idiotype of the antibody.

A paratope, also known as an antigen-binding site, is the part of an antibody which recognizes and binds to an antigen. It is a small region at the tip of the antibody's antigen-binding fragment and contains parts of the antibody's heavy and light chains. Each paratope is made up of six complementarity-determining regions - three from each of the light and heavy chains - that extend from a fold of anti-parallel beta sheets. Each arm of the Y-shaped antibody has an identical paratope at the end.

Complementarity-determining regions (CDRs) are part of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively, where these molecules bind to their specific antigen. A set of CDRs constitutes a paratope. As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes.
Anti-idiotypic vaccines comprise antibodies that have three-dimensional immunogenic regions, designated idiotopes, that consist of protein sequences that bind to cell receptors. Idiotopes are aggregated into idiotypes specific of their target antigen. An example of anti-idiotype antibody is Racotumomab.
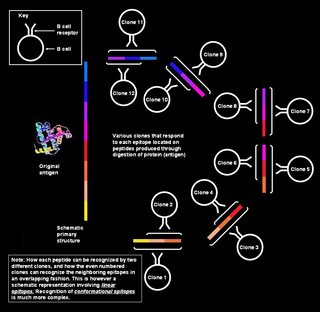
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.

Immunoglobulin class switching, also known as isotype switching, isotypic commutation or class-switch recombination (CSR), is a biological mechanism that changes a B cell's production of immunoglobulin from one type to another, such as from the isotype IgM to the isotype IgG. During this process, the constant-region portion of the antibody heavy chain is changed, but the variable region of the heavy chain stays the same. Since the variable region does not change, class switching does not affect antigen specificity. Instead, the antibody retains affinity for the same antigens, but can interact with different effector molecules.

In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.
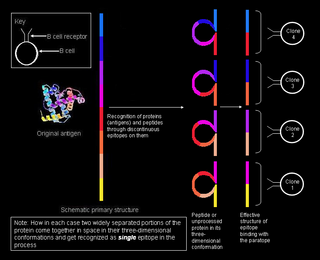
A conformational epitope is a sequence of sub-units composing an antigen that come in direct contact with a receptor of the immune system.
Immunodominance is the immunological phenomenon in which immune responses are mounted against only a few of the antigenic peptides out of the many produced. That is, despite multiple allelic variations of MHC molecules and multiple peptides presented on antigen presenting cells, the immune response is skewed to only specific combinations of the two. Immunodominance is evident for both antibody-mediated immunity and cell-mediated immunity. Epitopes that are not targeted or targeted to a lower degree during an immune response are known as subdominant epitopes. The impact of immunodominance is immunodomination, where immunodominant epitopes will curtail immune responses against non-dominant epitopes. Antigen-presenting cells such as dendritic cells, can have up to six different types of MHC molecules for antigen presentation. There is a potential for generation of hundreds to thousands of different peptides from the proteins of pathogens. Yet, the effector cell population that is reactive against the pathogen is dominated by cells that recognize only a certain class of MHC bound to only certain pathogen-derived peptides presented by that MHC class. Antigens from a particular pathogen can be of variable immunogenicity, with the antigen that stimulates the strongest response being the immunodominant one. The different levels of immunogenicity amongst antigens forms what is known as dominance hierarchy.
Immunology is the study of the immune system during health and disease. Below is a list of immunology-related articles.
Antigen-antibody interaction, or antigen-antibody reaction, is a specific chemical interaction between antibodies produced by B cells of the white blood cells and antigens during immune reaction. The antigens and antibodies combine by a process called agglutination. It is the fundamental reaction in the body by which the body is protected from complex foreign molecules, such as pathogens and their chemical toxins. In the blood, the antigens are specifically and with high affinity bound by antibodies to form an antigen-antibody complex. The immune complex is then transported to cellular systems where it can be destroyed or deactivated.











