
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year.

Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

The boron group are the chemical elements in group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the periodic table. The elements in the boron group are characterized by having three valence electrons. These elements have also been referred to as the triels.
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−.
Rubidium telluride is the inorganic compound with the formula Rb2Te. It is a yellow-green powder that melts at either 775 °C or 880 °C (two different values have been reported). It is an obscure material of minor academic interest.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Zinc phosphide (Zn3P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2).
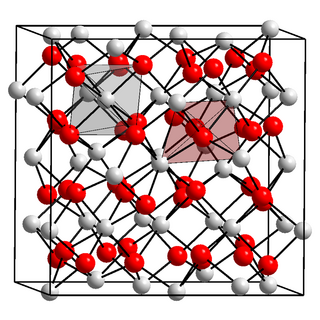
Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.

Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2O3. It is a gray solid that is insoluble in ordinary solvents.

Sodium telluride is the chemical compound with the formula Na2Te. This salt is the conjugate base of the thermally unstable acid hydrogen telluride, but it is usually prepared by reduction of tellurium with sodium. Na2Te is a challenging material to handle because it is very sensitive to air. Air oxidizes it initially to polytellurides, which have the formula Na2Tex (x > 1), and ultimately Te metal. Samples of Na2Te, which are colourless when absolutely pure, generally appear purple or dark gray due to the effects of air oxidation.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
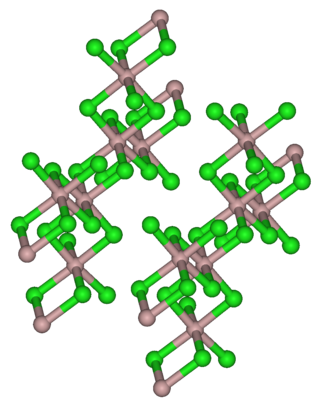
Indium(III) bromide, (indium tribromide), InBr3, is a chemical compound of indium and bromine. It is a Lewis acid and has been used in organic synthesis.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
Indium(III) selenide is a compound of indium and selenium. It has potential for use in photovoltaic devices and it has been the subject of extensive research. The two most common phases, α and β, have a layered structure, while γ is a "defect wurtzite structure." In all, five polymorpsare known: α, β, γ, δ, κ. The α- β phase transition is accompanied by a change in electrical conductivity. The band-gap of γ-In2Se3 is approximately 1.9 eV.

Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
Indium(III) hydroxide is the chemical compound with the formula In(OH)3. Its prime use is as a precursor to indium(III) oxide, In2O3. It is sometimes found as the rare mineral dzhalindite.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion [ReS4]− is possible.












