
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year.

A pnictogen is any of the chemical elements in group 15 of the periodic table. This group is also known as the nitrogen family. It consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and perhaps the chemically uncharacterized synthetic element moscovium (Mc).
An interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chlorides. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
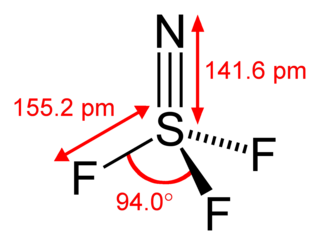
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula NSF3. It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds. It has tetrahedral molecular geometry around the sulfur atom, and is regarded to be a prime example of a compound that has a sulfur-nitrogen triple bond.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649°C.

Plutonium(III) fluoride or plutonium trifluoride is the chemical compound composed of plutonium and fluorine with the formula PuF3. This salt forms violet crystals. Plutonium(III) fluoride has the LaF3 structure where the coordination around the plutonium atoms is complex and usually described as tri-capped trigonal prismatic.
Trifluorides are compounds having three fluorine elements. Many metals form trifluorides, such as iron, the rare-earth elements, and the metals in the aluminium and scandium columns of the periodic table. Most metal trifluorides are poorly soluble in water except Ferric fluoride and Indium(III) fluoride, but several are soluble in other solvents.
Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.
Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Silver(III) fluoride, AgF3, is an unstable, bright-red, diamagnetic compound containing silver in the unusual +3 oxidation state. Its crystal structure is very similar to that of gold(III) fluoride: it is a polymer consisting of rectangular AgF4 units linked into chains by fluoro bridges.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.














