
Polonium is a chemical element with the symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though slightly longer-lived isotopes exist, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

Xenon hexafluoroplatinate is the product of the reaction of platinum hexafluoride with xenon, in an experiment that proved the chemical reactivity of the noble gases. This experiment was performed by Neil Bartlett at the University of British Columbia, who formulated the product as "Xe+[PtF6]−", although subsequent work suggests that Bartlett's product was probably a salt mixture and did not in fact contain this specific salt.
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 kg/m3 (22 lb/cu yd). It is one of the densest known gases under standard conditions. WF6 ls commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
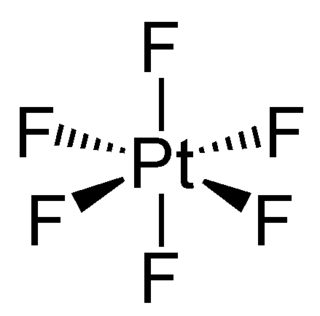
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.
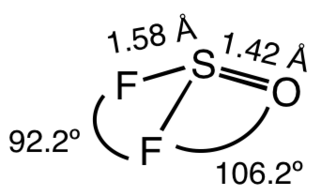
Thionyl fluoride is the inorganic compound with the formula SOF
2. This colourless gas is mainly of theoretical interest, but it is a product of the degradation of sulfur hexafluoride, an insulator in electrical equipment. The molecule adopts a distorted pyramidal structure, with Cs symmetry. The S-O and S-F distances are 1.42 and 1.58 Å, respectively. The O-S-F and F-S-F angles are 106.2 and 92.2°, respectively. Thionyl chloride and thionyl bromide have similar structures, although these compounds are liquid at room temperature. Mixed halides are also known, such as SOClF, thionyl chloride fluoride.
Tellurium hexafluoride is the inorganic compound of tellurium and fluorine with the chemical formula TeF6. It is a colorless, highly toxic gas with an unpleasant odor.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Nitrosonium octafluoroxenate(VI) is a chemical compound of xenon with nitrogen, oxygen, and fluorine, having formula (NO)
2XeF
8. It is an ionic compound containing well-separated nitrosonium cations (NO+) and octafluoroxenate(VI) anions (XeF2−
8). The molecular geometry of the octafluoroxenate(VI) ion is square antiprismatic, having Xe–F bond lengths of 1.971 Å, 1.946 Å, 1.958 Å, 2.052 Å, and 2.099 Å.
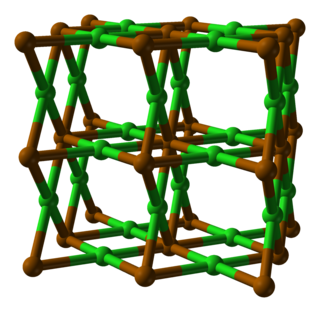
Polonium dichloride is a chemical compound of the radioactive metalloid, polonium and chlorine. Its chemical formula is PoCl2. It is an ionic salt.
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen. It is a volatile and very labile compound, from which many polonides can be derived. Additionally, like all polonium compounds, it is highly radioactive.
Polonium tetrachloride (also known as polonium(IV) chloride) is a chemical compound with the formula PoCl4. The salt is a hygroscopic bright yellow crystalline solid at room temperature. Above 200 °C, it tends to decompose into polonium dichloride and excess chlorine, similar to selenium tetrachloride and tellurium tetrachloride.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Polonium trioxide (also known as polonium(VI) oxide) is a chemical compound with the formula PoO3. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium dioxide (PoO2). It is an interchalcogen that has so far only been detected in trace amounts.
Polonium monoxide (also known as polonium(II) oxide or Poo) is a chemical compound with the formula PoO. It is one of three oxides of polonium, the other two being polonium dioxide (PoO2) and polonium trioxide (PoO3). It is an interchalcogen.
Polonium dibromide (also known as polonium(II) bromide) is a chemical compound with the formula PoBr2. This salt is a purple-brown crystalline solid at room temperature. It sublimes (decomposing slightly) at 110 °C/30 μ and decomposes when melted in nitrogen gas at 270–280 °C.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Polonium sulfide is an inorganic compound of polonium and sulfur with the chemical formula PoS. The compound is radioactive and forms black crystals.










