Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, formed when mixing liquid HF with liquid SbF5 in a 2:1 ratio. It is notable for its Lewis acidity and its ability to react with almost all known compounds.

Hydrogen fluoride is a chemical compound with the chemical formula HF. This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides.

Plutonium(IV) fluoride is a chemical compound with the formula (PuF4). It is a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants. Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.

Uranium pentafluoride is the inorganic compound with the chemical formula UF5. It is a pale yellow paramagnetic solid. The compound has attracted interest because it is related to uranium hexafluoride, which is widely used to produce uranium fuel. It crystallizes in two polymorphs, called α- and β-UF5.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Tantalum(V) fluoride is the inorganic compound with the formula TaF5. It is one of the principal molecular compounds of tantalum. Characteristic of some other pentafluorides, the compound is volatile but exists as an oligomer in the solid state.
Vanadium(IV) fluoride (VF4) is an inorganic compound of vanadium and fluorine. It is paramagnetic yellow-brown solid that is very hygroscopic. Unlike the corresponding vanadium tetrachloride, the tetrafluoride is not volatile because it adopts a polymeric structure. It decomposes before melting.

Gold(V) fluoride is the inorganic compound with the formula Au2F10. This fluoride compound features gold in its highest known oxidation state. This red solid dissolves in hydrogen fluoride but these solutions decompose, liberating fluorine.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.

Iridium hexafluoride, also iridium(VI) fluoride, (IrF6) is a compound of iridium and fluorine and one of the seventeen known binary hexafluorides. It is one of only a few compounds with iridium in the oxidation state +6.

Iridium(IV) fluoride is a chemical compound of iridium and fluorine, with the chemical formula IrF4 and is a dark brown solid. Early reports of IrF4 prior to 1965 are questionable and appear to describe the compound IrF5. The solid can be prepared by reduction of IrF5 with iridium black or reduction with H2 in aqueous HF The crystal structure of the solid is notable as it was the first example of a three-dimensional lattice structure found for a metal tetrafluoride and subsequently RhF4, PdF4 and PtF4 have been found to have the same structure. The structure has 6 coordinate, octahedral, iridium where two edges of the octahedra are shared and the two unshared fluorine atoms are cis to one another.

Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. The solid consists of tetramers [NbF5]4. It is a colorless solid that is rarely used.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Neptunium(V) fluoride or neptunium pentafluoride is a chemical compound of neptunium and fluorine with the formula NpF5.

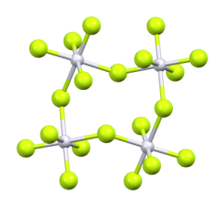
Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.