Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Karl Ernst Claus, a Russian-born scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, and in pyroxenite deposits in South Africa.

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless liquid is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
Ruthenium compounds are compounds containing the element ruthenium (Ru). Ruthenium compounds can have oxidation states ranging from 0 to +8, and −2. The properties of ruthenium and osmium compounds are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically.

Gold(V) fluoride is the inorganic compound with the formula Au2F10. This fluoride compound features gold in its highest known oxidation state. This red solid dissolves in hydrogen fluoride but these solutions decompose, liberating fluorine.

The dioxygenyl(or dioxyl) ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:
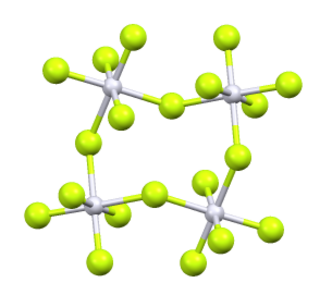
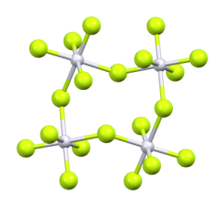
Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.

Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. It is a colorless solid.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Platinum pentafluoride is the inorganic compound with the empirical formula PtF5. This red volatile solid has rarely been studied but is of interest as one of the few binary fluorides of platinum, i.e., a compound containing only Pt and F. It is hydrolyzed in water.

Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.
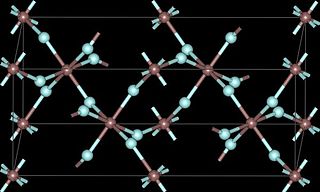
Ruthenium(III) fluoride is a fluoride of ruthenium, with the chemical formula of RuF3.
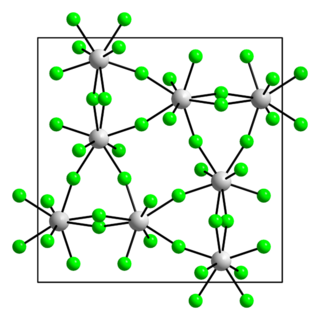
Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.

Ruthenium(IV) fluoride is a binary inorganic compound of ruthenium and fluorine with the formula RuF4.