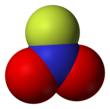
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bind to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.

Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.
In chemistry, a hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride, sulfur hexafluoride, chlorine trifluoride, the chlorite ion, and the triiodide ion are examples of hypervalent molecules.
The bond-dissociation energy is one measure of the strength of a chemical bond A−B. It can be defined as the standard enthalpy change when A−B is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K, although the enthalpy change at 298 K is also a frequently encountered parameter.
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short. Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname "FOOF" (a play on its chemical structure and its explosive tendencies).

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.
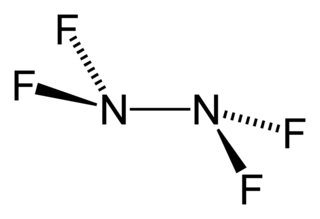
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, reactive inorganic gas. It is a fluorinated analog of hydrazine. It is a highly hazardous chemical that explodes in the presence of organic materials.

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:

The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry, and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. As such, fluoroalkanes like tetrafluoromethane are some of the most unreactive organic compounds.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.

In chemistry, chloryl refers to a triatomic cation with chemical formula ClO+
2. This species has the same general structure as chlorite (ClO−
2) but it is electronically different, with chlorine having a +5 oxidation state (rather than the +3 of chlorite). This makes it a rare example of a positively charged oxychloride. Chloryl compounds, such as FClO
2 and [ClO2][RuF6], are all highly reactive and react violently with water and most organic compounds.
Boron monofluoride or fluoroborylene is a chemical compound with formula BF, one atom of boron and one of fluorine. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.
Nitrogen pentafluoride (NF5) is a theoretical compound of nitrogen and fluorine that is hypothesized to exist based on the existence of the pentafluorides of the atoms below nitrogen in the periodic table, such as phosphorus pentafluoride. Theoretical models of the nitrogen pentafluoride molecule are either a trigonal bipyramidal covalently bound molecule with symmetry group D3h, or NF+
4F−, which would be an ionic solid.

Cyanogen fluoride is an inorganic linear compound which consists of a fluorine in a single bond with carbon, and a nitrogen in a triple bond with carbon. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. It is counted as an interhalogen compound, as the azide functional group is termed a pseudohalogen. It resembles ClN3, BrN3, and IN3 in this respect. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.
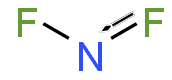
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride.
Difluoroamino sulfur pentafluoride is a gaseous chemical compound of fluorine, sulfur, and nitrogen. It is unusual in having a hexa-coordinated sulfur atom with a link to nitrogen. Other names for this substance include difluoro(pentafluorosulfur)amine, pentafluorosulfanyldifluoramine, and pentafluorosulfanyl N,N-difluoramine.
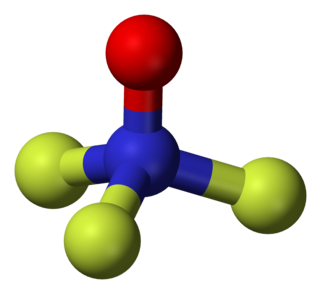
Trifluoramine oxide or Nitrogen trifluoride oxide (F3NO) is an inorganic molecule with strong fluorinating powers.