
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.

Biocon Limited is an Indian biopharmaceutical company based in Bangalore. It was founded by Kiran Mazumdar-Shaw in 1978. The company manufactures generic active pharmaceutical ingredients (APIs) that are sold in approximately 120 countries, including the United States and Europe. It also manufactures novel biologics as well as biosimilar insulins and antibodies, which are sold in India as branded formulations. Biocon's biosimilar products are also sold in both bulk and formulation forms in several emerging markets.

Kiran Mazumdar-Shaw is an Indian billionaire entrepreneur. She is the executive chairperson and founder of Biocon Limited and Biocon Biologics Limited, a biotechnology company based in Bangalore, India and the former chairperson of Indian Institute of Management, Bangalore. In 2014, she was awarded the Othmer Gold Medal for outstanding contributions to the progress of science and chemistry. She was on the Financial Times 2011 top 50 women in business list. In 2019, she was listed as the 68th most powerful woman in the world by Forbes. She was named EY World Entrepreneur Of The Year 2020. She is married to John Shaw.
Ustekinumab, sold under the brand name Stelara, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.

Aviptadil is an injectable synthetic formulation of human vasoactive intestinal peptide (VIP). VIP was discovered in 1970, and has been used to treat various inflammatory conditions, such as acute respiratory distress syndrome (ARDS), asthma, and chronic obstructive pulmonary disease (COPD).
Brodalumab, sold under the brand name Siliq in the US and Kyntheum in the EU, is a human monoclonal antibody designed for the treatment of inflammatory diseases.

Baricitinib, sold under the brand name Olumiant among others, is an immunomodulatory medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.
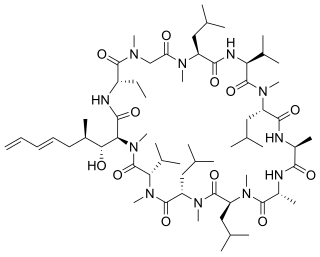
Voclosporin, sold under the brand name Lupkynis, is a calcineurin inhibitor used as an immunosuppressant medication for the treatment of lupus nephritis. It is an analog of ciclosporin that has enhanced action against calcineurin and greater metabolic stability.
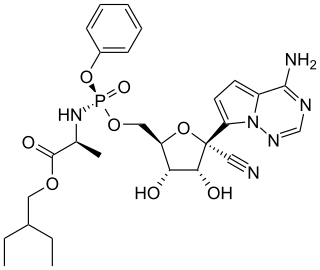
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries.
Viatris Inc. is an American global pharmaceutical and healthcare corporation headquartered in Canonsburg, Pennsylvania. The corporation was formed through the merger of Mylan and Upjohn, a legacy division of Pfizer, on November 16, 2020.

Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2. It is taken by mouth.

Casirivimab/imdevimab, sold under the brand name REGEN‑COV among others, is a combination medicine used for the treatment and prevention of COVID‑19. It consists of two human monoclonal antibodies, casirivimab and imdevimab that must be mixed together and administered as an infusion or subcutaneous injection. The combination of two antibodies is intended to prevent mutational escape. It is also available as a co-formulated product. It was developed by the American biotechnology company Regeneron Pharmaceuticals.

Covaxin is a whole inactivated virus-based COVID-19 vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research - National Institute of Virology.
Bamlanivimab/etesevimab is a combination of two monoclonal antibodies, bamlanivimab and etesevimab, administered together via intravenous infusion as a treatment for COVID-19. Both types of antibody target the surface spike protein of SARS‑CoV‑2.

Chloroquine and hydroxychloroquine are anti-malarial medications also used against some auto-immune diseases. Chloroquine, along with hydroxychloroquine, was an early experimental treatment for COVID-19. Neither drug prevents SARS-CoV-2 infection.

ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.
Sotrovimab, sold under the brand name Xevudy, is a human neutralizing monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. It was developed by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is designed to attach to the spike protein of SARS-CoV-2.

Nirmatrelvir/ritonavir, sold under the brand name Paxlovid, is a co-packaged medication used as a treatment for COVID‑19. It contains the antiviral medications nirmatrelvir and ritonavir and was developed by Pfizer. Both are protease inhibitors: nirmatrelvir inhibits SARS-CoV-2 main protease, while ritonavir inhibits HIV-1 protease, and is additionally a strong CYP3A inhibitor.














