
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides, is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.

Cutaneous T-cell lymphoma (CTCL) is a class of non-Hodgkin lymphoma, which is a type of cancer of the immune system. Unlike most non-Hodgkin lymphomas, CTCL is caused by a mutation of T cells. The cancerous T cells in the body initially migrate to the skin, causing various lesions to appear. These lesions change shape as the disease progresses, typically beginning as what appears to be a rash which can be very itchy and eventually forming plaques and tumors before spreading to other parts of the body.

Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for non-Hodgkin's lymphoma. The drug uses the monoclonal mouse IgG1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.

Sézary disease, or Sézary syndrome, is a type of cutaneous T-cell lymphoma that was first described by Albert Sézary. The affected T cells, known as Sézary's cells or Lutzner cells, have pathological quantities of mucopolysaccharides. Sézary disease is sometimes considered a late stage of mycosis fungoides with lymphadenopathy.
Tositumomab is a murine monoclonal antibody which targets the CD20 antigen produced in mammalian cell. It was combined with iodine-131 to produce a radiopharmaceutical for unsealed source radiotherapy, Iodine-131 Tositumomab, for the treatment of non-Hodgkins lymphoma. It is classified as a IgG2a lambda antibody.
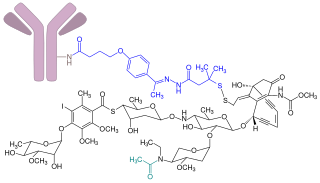
Inotuzumab ozogamicin, sold under the brand name Besponsa, is an antibody-drug conjugate medication used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia. It is administered by intravenous infusion.
Blinatumomab, sold under the brand name Blincyto, and known informally as blina, is a biopharmaceutical medication used as a second-line treatment for Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia. It belongs to a class of constructed monoclonal antibodies, bi-specific T-cell engagers (BiTEs), that exert action selectively and direct the human immune system to act against tumor cells. Blinatumomab specifically targets the CD19 antigen present on B cells. In December 2014, it was approved by the US Food and Drug Administration under the accelerated approval program; marketing authorization depended on the outcome of clinical trials that were ongoing at the time of approval. Blinatumomab is given via intravenous infusion.
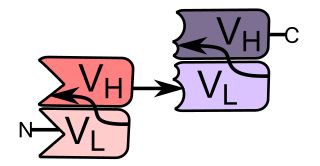
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG.
Obinutuzumab, sold under the brand name Gazyva among others, is a humanized anti-CD20 monoclonal antibody used as a treatment for cancer. It was originated by GlycArt Biotechnology AG and developed by Roche.
MorphoSys AG is a German biopharmaceutical company founded in 1992. The company is headquartered near Munich, Germany, and has a wholly owned subsidiary, MorphoSys US Inc., in Boston, Massachusetts, in the US. The company has various antibody, protein and peptide technologies that it uses to discover and develop both proprietary and partnered drug candidates. The company has more than 100 drugs in its wider pipeline that are being investigated for a variety of diseases. While many of these are being developed in partnership with pharma and biotech companies, MorphoSys also has a proprietary pipeline with a focus on cancer and autoimmune diseases.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seagen in the US.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
Polatuzumab vedotin, sold under the brand name Polivy, is a CD79b-directed antibody-drug conjugate medication used for the treatment of diffuse large B-cell lymphoma (cancer). It was developed by the Genentech subsidiary of Roche.
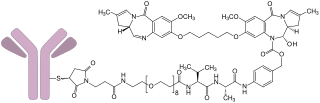
Loncastuximab tesirine, sold under the brand name Zynlonta, is a monoclonal antibody conjugate medication used to treat large B-cell lymphoma and high-grade B-cell lymphoma. It is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19.

Selinexor sold under the brand name Xpovio among others, is a selective inhibitor of nuclear export used as an anti-cancer medication. It works by blocking the action of exportin 1 and thus blocking the transport of several proteins involved in cancer-cell growth from the cell nucleus to the cytoplasm, which ultimately arrests the cell cycle and leads to apoptosis. It is the first drug with this mechanism of action.

Zanubrutinib, sold under the brand name Brukinsa, is an anticancer medication used for the treatment of mantle cell lymphoma (MCL), Waldenström's macroglobulinemia (WM), marginal zone lymphoma (MZL), and chronic lymphocytic leukemia (CLL). Zanubrutinib is classified as a Bruton's tyrosine kinase (BTK) inhibitor. It is given by mouth.
Tafasitamab, sold under the brand name Monjuvi, is a medication used in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
BeiGene, Ltd. is a China-based drug developer. It specializes in the development of drugs for cancer treatment. Founded in 2010 by chief executive officer John V. Oyler and Xiaodong Wang, the multinational company headquartered in Cambridge, Massachusetts has offices in North America, Europe, South America, Asia and Australia. BeiGene has a large presence in Chinese market. BeiGene has developed several pharmaceuticals, including tislelizumab, a checkpoint inhibitor, and zanubrutinib, a Bruton's tyrosine kinase inhibitor.
Belantamab mafodotin, sold under the brand name Blenrep, is a medication for the treatment of relapsed and refractory multiple myeloma.
Mosunetuzumab, sold under the brand name Lunsumio, is a monoclonal antibody used for the treatment of follicular lymphoma. It bispecifically binds CD20 and CD3 to engage T-cells. It was developed by Genentech.








