Anaplastic large-cell lymphoma (ALCL) refers to a group of non-Hodgkin lymphomas in which aberrant T cells proliferate uncontrollably. Considered as a single entity, ALCL is the most common type of peripheral lymphoma and represents ~10% of all peripheral lymphomas in children. The incidence of ALCL is estimated to be 0.25 cases per 100,000 people in the United States of America. There are four distinct types of anaplastic large-cell lymphomas that on microscopic examination share certain key histopathological features and tumor marker proteins. However, the four types have very different clinical presentations, gene abnormalities, prognoses, and/or treatments.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
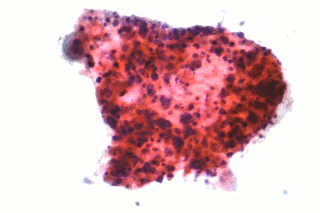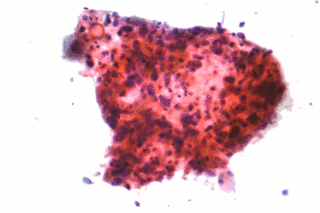
Non-small-cell lung cancer (NSCLC), or non-small-cell lung carcinoma, is any type of epithelial lung cancer other than small-cell lung cancer (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively and postoperatively.

Anaplastic lymphoma kinase (ALK) also known as ALK tyrosine kinase receptor or CD246 is an enzyme that in humans is encoded by the ALK gene.

Proto-oncogene tyrosine-protein kinase ROS is an enzyme that in humans is encoded by the ROS1 gene.

Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC). Crizotinib inhibits the c-Met/Hepatocyte growth factor receptor (HGFR) tyrosine kinase, which is involved in the oncogenesis of a number of other histological forms of malignant neoplasms. It also acts as an ALK and ROS1 inhibitor.

ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.

ALK positive lung cancer is a primary malignant lung tumor whose cells contain a characteristic abnormal configuration of DNA wherein, most frequently, the echinoderm microtubule-associated protein-like 4 (EML4) gene is fused to the anaplastic lymphoma kinase (ALK) gene. Less frequently, there will be novel translocation partners for the ALK gene, in place of EML4. This abnormal gene fusion leads to the production of a protein that appears, in many cases, to promote and maintain the malignant behavior of the cancer cells.

Brigatinib, sold under the brand name Alunbrig among others, is a small-molecule targeted cancer therapy being developed by Ariad Pharmaceuticals, Inc. Brigatinib acts as both an anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.

Inflammatory myofibroblastic tumor (IMT) is a rare neoplasm of the mesodermal cells that form the connective tissues which support virtually all of the organs and tissues of the body. IMT was formerly termed inflammatory pseudotumor. Currently, however, inflammatory pseudotumor designates a large and heterogeneous group of soft tissue tumors that includes inflammatory myofibroblastic tumor, plasma cell granuloma, xanthomatous pseudotumor, solitary mast cell granuloma, inflammatory fibrosarcoma, pseudosarcomatous myofibroblastic proliferation, myofibroblastoma, inflammatory myofibrohistiocytic proliferation, and other tumors that develop from connective tissue cells. Inflammatory pseudotumour is a generic term applied to various neoplastic and non-neoplastic tissue lesions which share a common microscopic appearance consisting of spindle cells and a prominent presence of the white blood cells that populate chronic or, less commonly, acute inflamed tissues.
Targeted molecular therapy for neuroblastoma involves treatment aimed at molecular targets that have a unique expression in this form of cancer. Neuroblastoma, the second most common pediatric malignant tumor, often involves treatment through intensive chemotherapy. A number of molecular targets have been identified for the treatment of high-risk forms of this disease. Aiming treatment in this way provides a more selective way to treat the disease, decreasing the risk for toxicities that are associated with the typical treatment regimen. Treatment using these targets can supplement or replace some of the intensive chemotherapy that is used for neuroblastoma. These molecular targets of this disease include GD2, ALK, and CD133. GD2 is a target of immunotherapy, and is the most fully developed of these treatment methods, but is also associated with toxicities. ALK has more recently been discovered, and drugs in development for this target are proving to be successful in neuroblastoma treatment. The role of CD133 in neuroblastoma has also been more recently discovered and is an effective target for treatment of this disease.
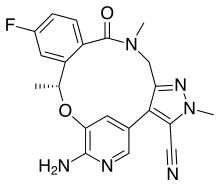
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014.

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.

Alectinib (INN), sold under the brand name Alecensa, is an anticancer medication that is used to treat non-small-cell lung cancer (NSCLC). It blocks the activity of anaplastic lymphoma kinase (ALK). It is taken by mouth. It was developed by Chugai Pharmaceutical Co. Japan, which is part of the Hoffmann-La Roche group.
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).
Christine M. Lovly is an associate professor of medicine at Vanderbilt University. Her research involves the development of novel treatment strategies for ALK positive lung cancer.

Selpercatinib, sold under the brand name Retevmo among others, is a medication for the treatment of cancers in people whose tumors have an alteration in a specific gene. It is taken by mouth.

Repotrectinib, sold under the brand name Augtyro, is an anti-cancer medication used for the treatment of non-small cell lung cancer. It is taken by mouth. Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 (ROS1) and of the tropomyosin receptor tyrosine kinases (TRKs) TRKA, TRKB, and TRKC.