Antiphospholipid syndrome, or antiphospholipid antibody syndrome, is an autoimmune, hypercoagulable state caused by antiphospholipid antibodies. APS can lead to blood clots (thrombosis) in both arteries and veins, pregnancy-related complications, and other symptoms like low platelets, kidney disease, heart disease, and rash. Although the exact etiology of APS is still not clear, genetics is believed to play a key role in the development of the disease. Diagnosis is made based on symptoms and testing, but sometimes research criteria are used to aid in diagnosis. The research criteria for definite APS requires one clinical event and two positive blood test results spaced at least three months apart that detect lupus anticoagulant, anti-apolipoprotein antibodies, and/or anti-cardiolipin antibodies.

Vasculitis is a group of disorders that destroy blood vessels by inflammation. Both arteries and veins are affected. Lymphangitis is sometimes considered a type of vasculitis. Vasculitis is primarily caused by leukocyte migration and resultant damage. Although both occur in vasculitis, inflammation of veins (phlebitis) or arteries (arteritis) on their own are separate entities.

Panniculitis is a group of diseases whose hallmark is inflammation of subcutaneous adipose tissue. Symptoms include tender skin nodules, and systemic signs such as weight loss and fatigue.

Lichen planus (LP) is a chronic inflammatory and autoimmune disease that affects the skin, nails, hair, and mucous membranes. It is not an actual lichen, but is named for its appearance. It is characterized by polygonal, flat-topped, violaceous papules and plaques with overlying, reticulated, fine white scale, commonly affecting dorsal hands, flexural wrists and forearms, trunk, anterior lower legs and oral mucosa. The hue may be gray-brown in people with darker skin. Although there is a broad clinical range of LP manifestations, the skin and oral cavity remain as the major sites of involvement. The cause is unknown, but it is thought to be the result of an autoimmune process with an unknown initial trigger. There is no cure, but many different medications and procedures have been used in efforts to control the symptoms.

Thromboangiitis obliterans, also known as Buerger disease or Winiwarter-Buerger disease, is a recurring progressive inflammation and thrombosis (clotting) of small and medium arteries and veins of the hands and feet. It is strongly associated with use of tobacco products, primarily from smoking, but is also associated with smokeless tobacco.

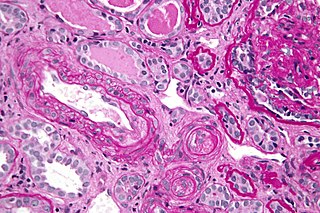
Polyarteritis nodosa (PAN) is a systemic necrotizing inflammation of blood vessels (vasculitis) affecting medium-sized muscular arteries, typically involving the arteries of the kidneys and other internal organs but generally sparing the lungs' circulation. Small aneurysms are strung like the beads of a rosary, therefore making this "rosary sign" an important diagnostic feature of the vasculitis. PAN is sometimes associated with infection by the hepatitis B or hepatitis C virus. The condition may be present in infants.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Livedo reticularis is a common skin finding consisting of a mottled reticulated vascular pattern that appears as a lace-like purplish discoloration of the skin. The discoloration is caused by reduction in blood flow through the arterioles that supply the cutaneous capillaries, resulting in deoxygenated blood showing as blue discoloration. This can be a secondary effect of a condition that increases a person's risk of forming blood clots, including a wide array of pathological and nonpathological conditions. Examples include hyperlipidemia, microvascular hematological or anemia states, nutritional deficiencies, hyper- and autoimmune diseases, and drugs/toxins.
Catastrophic antiphospholipid syndrome (CAPS), also known as Asherson's syndrome, is a rare autoimmune disease in which widespread, intravascular clotting causes multi-organ failure. The syndrome is caused by antiphospholipid antibodies that target a group of proteins in the body that are associated with phospholipids. These antibodies activate endothelial cells, platelets, and immune cells, ultimately causing a large inflammatory immune response and widespread clotting. CAPS was first described by Ronald Asherson in 1992. The syndrome exhibits thrombotic microangiopathy, multiple organ thromboses, and in some cases tissue necrosis and is considered an extreme or catastrophic variant of the antiphospholipid syndrome.

Sneddon's syndrome is a form of arteriopathy characterized by several symptoms, including:
Anetoderma is a benign but uncommon disorder that causes localized areas of flaccid or herniated sac-like skin due to a focal reduction of dermal elastic tissue. Anetoderma is subclassified as primary anetoderma, secondary anetoderma, iatrogenic anetoderma of prematurity, congenital anetoderma, familial anetoderma, and drug-induced anetoderma.
Rheumatoid vasculitis is skin condition that is a typical feature of rheumatoid arthritis, presenting as peripheral vascular lesions that are localized purpura, cutaneous ulceration, and gangrene of the distal parts of the extremities.

Necrotizing vasculitis, also called systemic necrotizing vasculitis, is a general term for the inflammation of veins and arteries that develops into necrosis and narrows the vessels.
Cryofibrinogenemia refers to a condition classified as a fibrinogen disorder in which a person's blood plasma is allowed to cool substantially, causing the (reversible) precipitation of a complex containing fibrinogen, fibrin, fibronectin, and, occasionally, small amounts of fibrin split products, albumin, immunoglobulins and other plasma proteins.

Cutaneous small-vessel vasculitis (CSVV), is inflammation of small blood vessels, usually accompanied by small lumps beneath the skin. The condition is also known as hypersensitivity vasculitis, cutaneous leukocytoclastic vasculitis, hypersensitivity angiitis, cutaneous leukocytoclastic angiitis, cutaneous necrotizing vasculitis and cutaneous necrotizing venulitis,

Majocchi's granuloma is a skin condition characterized by deep, pustular plaques, and is a form of tinea corporis. It is a localized form of fungal folliculitis. Lesions often have a pink and scaly central component with pustules or folliculocentric papules at the periphery. The name comes from Domenico Majocchi, who discovered the disorder in 1883. Majocchi was a professor of dermatology at the University of Parma and later the University of Bologna. The most common dermatophyte is called Trichophyton rubrum.
Blood clots are a relatively common occurrence in the general population and are seen in approximately 1-2% of the population by age 60. Typically, blood clots develop in the deep veins of the lower extremities, deep vein thrombosis (DVT) or as a blood clot in the lung, pulmonary embolism. A very small number of people who develop blood clots have a more serious and often life-threatening condition, known as thrombotic storm (TS). TS is characterized by the development of more than one blood clot in a short period of time. These clots often occur in multiple and sometimes unusual locations in the body and are often difficult to treat. TS may be associated with an existing condition or situation that predisposes a person to blood clots, such as injury, infection, or pregnancy. In many cases, a risk assessment will identify interventions that will prevent the formation of blood clots.
Vasculitic neuropathy is a peripheral neuropathic disease. In a vasculitic neuropathy there is damage to the vessels that supply blood to the nerves. It can be as part of a systemic problem or can exist as a single-organ issue only affecting the peripheral nervous system (PNS). It is diagnosed with the use of electrophysiological testing, blood tests, nerve biopsy and clinical examination. It is a serious medical condition that can cause prolonged morbidity and disability and generally requires treatment. Treatment depends on the type but it is mostly with corticosteroids or immunomodulating therapies.
Cutaneous manifestations of COVID-19 are characteristic signs or symptoms of the Coronavirus disease 2019 that occur in the skin. The American Academy of Dermatology reports that skin lesions such as morbilliform, pernio, urticaria, macular erythema, vesicular purpura, papulosquamous purpura and retiform purpura are seen in people with COVID-19. Pernio-like lesions were more common in mild disease while retiform purpura was seen only in critically ill patients. The major dermatologic patterns identified in individuals with COVID-19 are urticarial rash, confluent erythematous/morbilliform rash, papulovesicular exanthem, chilbain-like acral pattern, livedo reticularis and purpuric "vasculitic" pattern. Chilblains and Multisystem inflammatory syndrome in children are also cutaneous manifestations of COVID-19.
Retiform purpura is the result of total vascular blockage and damage to the skin's blood vessels. The skin then shows lesions, appearing due to intravascular issues where clots, proteins, or emboli block skin vessels. They can also result from direct harm to the vessel walls, as seen in conditions like vasculitis, calciphylaxis, and certain severe opportunistic infections.












