
Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important role while growing superlattice structures.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.
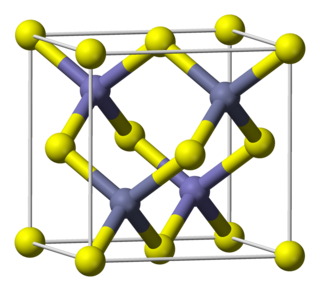
Indium antimonide (InSb) is a crystalline compound made from the elements indium (In) and antimony (Sb). It is a narrow-gap semiconductor material from the III-V group used in infrared detectors, including thermal imaging cameras, FLIR systems, infrared homing missile guidance systems, and in infrared astronomy. Indium antimonide detectors are sensitive to infrared wavelengths between 1 and 5 μm.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment but applications are declining because of environmental concerns

Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F). ZnSe occurs as the rare mineral stilleite, named after Hans Stille.
Indium gallium arsenide (InGaAs) is a ternary alloy of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are group III elements of the periodic table while arsenic is a group V element. Alloys made of these chemical groups are referred to as "III-V" compounds. InGaAs has properties intermediate between those of GaAs and InAs. InGaAs is a room-temperature semiconductor with applications in electronics and photonics.

Magnesium sulfide is an inorganic compound with the formula MgS. It is a white crystalline material but often is encountered in an impure form that is brown and non-crystalline powder. It is generated industrially in the production of metallic iron.

Copper indium gallium (di)selenide (CIGS) is a I-III-VI2 semiconductor material composed of copper, indium, gallium, and selenium. The material is a solid solution of copper indium selenide (often abbreviated "CIS") and copper gallium selenide. It has a chemical formula of CuIn1−xGaxSe2, where the value of x can vary from 0 (pure copper indium selenide) to 1 (pure copper gallium selenide). CIGS is a tetrahedrally bonded semiconductor, with the chalcopyrite crystal structure, and a bandgap varying continuously with x from about 1.0 eV (for copper indium selenide) to about 1.7 eV (for copper gallium selenide).

Zinc telluride is a binary chemical compound with the formula ZnTe. This solid is a semiconductor material with a direct band gap of 2.26 eV. It is usually a p-type semiconductor. Its crystal structure is cubic, like that for sphalerite and diamond.

Mercury selenide is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
In chemistry, the Grimm–Sommerfeld rule predicts that binary compounds with covalent character that have an average of 4 electrons per atom will have structures where both atoms are tetrahedrally coordinated. Examples are silicon carbide, the III-V semiconductors indium phosphide and gallium arsenide, the II-VI semiconductors, cadmium sulfide, cadmium selenide.
Selective area epitaxy is the local growth of epitaxial layer through a patterned amorphous dielectric mask (typically SiO2 or Si3N4) deposited on a semiconductor substrate. Semiconductor growth conditions are selected to ensure epitaxial growth on the exposed substrate, but not on the dielectric mask. SAE can be executed in various epitaxial growth methods such as molecular beam epitaxy (MBE), metalorganic vapour phase epitaxy (MOVPE) and chemical beam epitaxy (CBE). By SAE, semiconductor nanostructures such as quantum dots and nanowires can be grown to their designed places.

I-III-VI2 semiconductors are solid semiconducting materials that contain three or more chemical elements belonging to groups I, III and VI (IUPAC groups 1/11, 13 and 16) of the periodic table. They usually involve two metals and one chalcogen. Some of these materials have a direct bandgap, Eg, of approximately 1.5 eV, which makes them efficient absorbers of sunlight and thus potential solar cell materials. A fourth element is often added to a I-III-VI2 material to tune the bandgap for maximum solar cell efficiency. A representative example is copper indium gallium selenide (CuInxGa(1–x)Se2, Eg = 1.7–1.0 eV for x = 0–1), which is used in copper indium gallium selenide solar cells.
II-VI semiconductor compounds are compounds composed of a metal from either group 2 or 12 of the periodic table and a nonmetal from group 16 . These semiconductors crystallize either in the zincblende lattice structure or the wurtzite crystal structure. They generally exhibit large band gaps, making them popular for short wavelength applications in optoelectronics.
Aluminium gallium antimonide, also known as gallium aluminium antimonide or AlGaSb (AlxGa1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium antimonide and gallium antimonide. The alloy can contain any ratio between aluminium and gallium. AlGaSb refers generally to any composition of the alloy.
Gallium indium antimonide, also known as indium gallium antimonide, GaInSb, or InGaSb (GaxIn1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between gallium antimonide and indium antimonide. The alloy can contain any ratio between gallium and indium. GaInSb refers generally to any composition of the alloy.
Aluminium arsenide antimonide, or AlAsSb (AlAs1-xSbx), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium arsenide and aluminium antimonide. The alloy can contain any ratio between arsenic and antimony. AlAsSb refers generally to any composition of the alloy.










