
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to form an alkene using a titanium chloride compound such as titanium(III) chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagents. Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium, zinc, and magnesium. This reaction is related to the Pinacol coupling reaction which also proceeds by reductive coupling of carbonyl compounds.
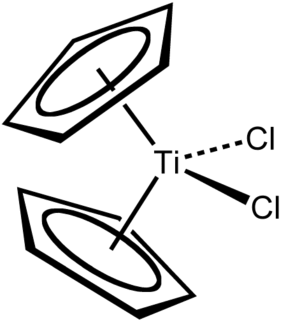
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.

Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry.

Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.

Dicarbonylbis(cyclopentadienyl)titanium is the chemical compound with the formula (η5-C5H5)2Ti(CO)2, abbreviated Cp2Ti(CO)2. This maroon-coloured, air-sensitive species is soluble in aliphatic and aromatic solvents. It has been used for the deoxygenation of sulfoxides, reductive coupling of aromatic aldehydes and reduction of aldehydes.
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.

Organozirconium compounds are organometallic compounds containing a carbon to zirconium chemical bond. Organozirconium chemistry is the corresponding science exploring properties, structure, and reactivity of these compounds. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that lead to Richard R. Schrock's Nobel Prize discovery in olefin metathesis.
Niobium(III) chloride also known as niobium trichloride is a compound of niobium and chlorine. The binary phase NbCl3 is not well characterized but many adducts are known.
In chemistry, alpha elimination refers to particular types of elimination reactions. The definition of alpha elimination differs for organometallic and organic chemistry.
![Structure of [Li(OEt2)3] [NbMe6] . POZJAP.png](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c6/POZJAP.png/330px-POZJAP.png)



![Nb(III) catalyzed formal [2+2+2] cycloadditions NbIII 222s.png](http://upload.wikimedia.org/wikipedia/commons/9/92/NbIII_222s.png)














