
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

A drop or droplet is a small column of liquid, bounded completely or almost completely by free surfaces. A drop may form when liquid accumulates at the end of a tube or other surface boundary, producing a hanging drop called a pendant drop. Drops may also be formed by the condensation of a vapor or by atomization of a larger mass of solid. Water vapor will condense into droplets depending on the temperature. The temperature at which droplets form is called the dew point.

An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be generated from natural or human causes. The term aerosol commonly refers to the mixture of particulates in air, and not to the particulate matter alone. Examples of natural aerosols are fog, mist or dust. Examples of human caused aerosols include particulate air pollutants, mist from the discharge at hydroelectric dams, irrigation mist, perfume from atomizers, smoke, dust, sprayed pesticides, and medical treatments for respiratory illnesses.

In chemistry, electrophoresis is the motion of charged dispersed particles or dissolved charged molecules relative to a fluid under the influence of a spatially uniform electric field. As a rule, these are zwitterions. Electrophoresis of positively charged particles or molecules (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles or molecules (anions) is sometimes called anaphoresis..
Sedimentation equilibrium in a suspension of different particles, such as molecules, exists when the rate of transport of each material in any one direction due to sedimentation equals the rate of transport in the opposite direction due to diffusion. Sedimentation is due to an external force, such as gravity or centrifugal force in a centrifuge.
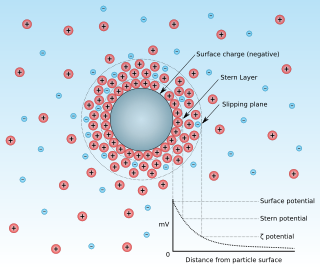
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
The DLVO theory explains the aggregation and kinetic stability of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,
Soil texture is a classification instrument used both in the field and laboratory to determine soil classes based on their physical texture. Soil texture can be determined using qualitative methods such as texture by feel, and quantitative methods such as the hydrometer method based on Stokes' law. Soil texture has agricultural applications such as determining crop suitability and to predict the response of the soil to environmental and management conditions such as drought or calcium (lime) requirements. Soil texture focuses on the particles that are less than two millimeters in diameter which include sand, silt, and clay. The USDA soil taxonomy and WRB soil classification systems use 12 textural classes whereas the UK-ADAS system uses 11. These classifications are based on the percentages of sand, silt, and clay in the soil.
The equivalent spherical diameter of an irregularly shaped object is the diameter of a sphere of equivalent geometric, optical, electrical, aerodynamic or hydrodynamic behavior to that of the particle under investigation.

In granulometry, the particle-size distribution (PSD) of a powder, or granular material, or particles dispersed in fluid, is a list of values or a mathematical function that defines the relative amount, typically by mass, of particles present according to size. Significant energy is usually required to disintegrate soil, etc. particles into the PSD that is then called a grain size distribution.
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. The fluid might be a simple Newtonian liquid, or complex heterogeneous dispersion, emulsion or even a porous body. There are several different electroacoustic effects depending on the nature of the fluid.
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Particles can be solid, liquid or gas bubbles with sizes on the scale of a micrometer or nanometer. There is a common source of all these effects—the so-called interfacial 'double layer' of charges. Influence of an external force on the diffuse layer generates tangential motion of a fluid with respect to an adjacent charged surface. This force might be electric, pressure gradient, concentration gradient, or gravity. In addition, the moving phase might be either continuous fluid or dispersed phase.

Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with colloids, heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a true solution and a suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane.
Sedimentation potential occurs when dispersed particles move under the influence of either gravity or centrifugation or electricity in a medium. This motion disrupts the equilibrium symmetry of the particle's double layer. While the particle moves, the ions in the electric double layer lag behind due to the liquid flow. This causes a slight displacement between the surface charge and the electric charge of the diffuse layer. As a result, the moving particle creates a dipole moment. The sum of all of the dipoles generates an electric field which is called sedimentation potential. It can be measured with an open electrical circuit, which is also called sedimentation current.
Particle size analysis, particle size measurement, or simply particle sizing, is the collective name of the technical procedures, or laboratory techniques which determines the size range, and/or the average, or mean size of the particles in a powder or liquid sample.

Laser diffraction analysis, also known as laser diffraction spectroscopy, is a technology that utilizes diffraction patterns of a laser beam passed through any object ranging from nanometers to millimeters in size to quickly measure geometrical dimensions of a particle. This particle size analysis process does not depend on volumetric flow rate, the amount of particles that passes through a surface over time.
The Stöber process is a chemical process used to prepare silica particles of controllable and uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism – a particle aggregation model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.
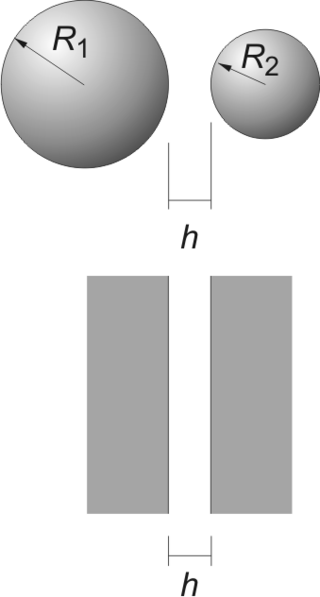
The Derjaguin approximation (or sometimes also referred to as the proximity approximation), named after the Russian scientist Boris Derjaguin, expresses the force profile acting between finite size bodies in terms of the force profile between two planar semi-infinite walls. This approximation is widely used to estimate forces between colloidal particles, as forces between two planar bodies are often much easier to calculate. The Derjaguin approximation expresses the force F(h) between two bodies as a function of the surface separation as
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of depletants, which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase.
Dispersion Technology Inc is a scientific instrument manufacturer located in Bedford Hills, New York. It was founded in 1996 by Philip Goetz and Dr. Andrei Dukhin. The company develops and sells analytical instruments intended for characterizing concentrated dispersions and emulsions, complying with the International Standards for acoustic particle sizing ISO 20998 and electroacoustic zeta potential measurement ISO 13099.


















