Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

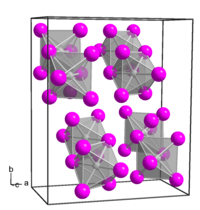
Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.
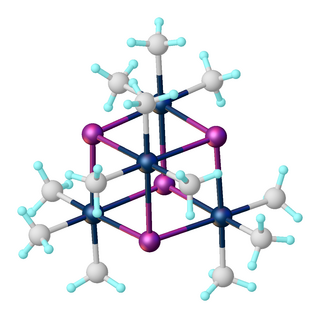
Trimethylplatinum iodide is the organoplatinum complex with the formula [(CH3)3PtI]4. It is a white, air-stable solid that was one of the first σ-alkyl metal complexes reported. It arises from the reaction of potassium hexachloroplatinate with methylmagnesium iodide. The complex exists as a tetramer: a cubane-type cluster with four octahedral Pt(IV) centers linked by four iodides as triply bridging ligands. Due to its stability, it is often utilized as a precursor en route to the synthesis of other organoplatinum compound, such as hydrosilylation catalysts. It is also used as a precursor for forming platinum layers for electronics.
Samarium(III) iodide is an inorganic compound, a salt of samarium and hydroiodic acid with the chemical formula SmI
3.
Platinum-samarium is a binary inorganic compound of platinum and samarium with the chemical formula PtSm. This intermetallic compound forms crystals.

Chromium(II) sulfide is an inorganic compound of chromium and sulfur with the chemical formula CrS. The compound forms black hexagonal crystals, insoluble in water.
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4. The compound forms volatile black crystals.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.
Californium(II) iodide is a binary inorganic compound of californium and iodine with the formula CfI
2.

Platinum(II) iodide is a binary inorganic compound of platinum and iodine with the chemical formula PtI
2.
Iridium(IV) iodide is a binary chemical compound of iridium and iodide with the chemical formula IrI
4.

Niobium(IV) iodide is an iodide of niobium, with the chemical formula of NbI4.
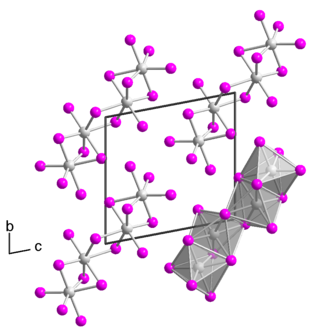
Tantalum(IV) iodide is an inorganic compound with the chemical formula TaI4. It dissolves in water to give a green solution, but the color fades when left in the air and produces a white precipitate.
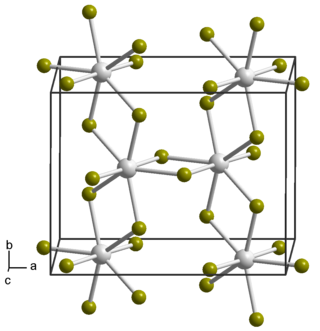
Neptunium tetrabromide is a binary inorganic compound of neptunium metal and bromine with the chemical formula NpBr4.

Protactinium tetrafluoride is a binary inorganic compound of protactinium metal and fluorine with the chemical formula PaF4.
Protactinium tetraiodide is a binary inorganic compound of protactinium metal and iodine with the chemical formula PaI4.