
Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidism. Some, however, use the terms interchangeably. Signs and symptoms vary between people and may include irritability, muscle weakness, sleeping problems, a fast heartbeat, heat intolerance, diarrhea, enlargement of the thyroid, hand tremor, and weight loss. Symptoms are typically less severe in the elderly and during pregnancy. An uncommon but life-threatening complication is thyroid storm in which an event such as an infection results in worsening symptoms such as confusion and a high temperature; this often results in death. The opposite is hypothyroidism, when the thyroid gland does not make enough thyroid hormone.

The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the isthmus (pl.: isthmi). The thyroid gland is a butterfly-shaped gland located in the neck below the Adam's apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones – triiodothyronine (T3) and thyroxine (T4) – and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis and growth and development in children. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.

Graves' disease, also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyroid. Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss. Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves' ophthalmopathy. About 25 to 30% of people with the condition develop eye problems.

Iodothyronine deiodinases (EC 1.21.99.4 and EC 1.21.99.3) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor, influences the expression of genes in practically every vertebrate cell. Iodothyronine deiodinases are unusual in that these enzymes contain selenium, in the form of an otherwise rare amino acid selenocysteine.
Thyroid-stimulating hormone (also known as thyrotropin, thyrotropic hormone, or abbreviated TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroxine (T4), and then triiodothyronine (T3) which stimulates the metabolism of almost every tissue in the body. It is a glycoprotein hormone produced by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid.
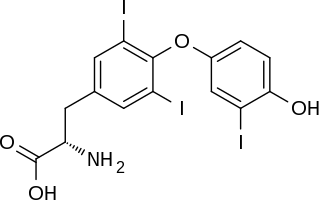
Triiodothyronine, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate.

Levothyroxine, also known as L-thyroxine, is a synthetic form of the thyroid hormone thyroxine (T4). It is used to treat thyroid hormone deficiency (hypothyroidism), including a severe form known as myxedema coma. It may also be used to treat and prevent certain types of thyroid tumors. It is not indicated for weight loss. Levothyroxine is taken orally (by mouth) or given by intravenous injection. Levothyroxine has a half-life of 7.5 days when taken daily, so about six weeks is required for it to reach a steady level in the blood.

The Wolff–Chaikoff effect is a presumed reduction in thyroid hormone levels caused by ingestion of a large amount of iodine.

Thiamazole, also known as methimazole, is a medication used to treat hyperthyroidism. This includes Graves disease, toxic multinodular goiter, and thyrotoxic crisis. It is taken by mouth. Full effects may take a few weeks to occur.
Thyroid disease is a medical condition that affects the function of the thyroid gland. The thyroid gland is located at the front of the neck and produces thyroid hormones that travel through the blood to help regulate many other organs, meaning that it is an endocrine organ. These hormones normally act in the body to regulate energy use, infant development, and childhood development.
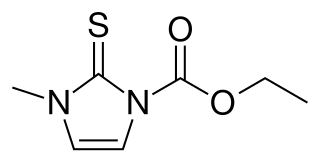
Carbimazole (brand names Neo-Mercazole, Anti-Thyrox, etc.) is used to treat hyperthyroidism. Carbimazole is a pro-drug as after absorption it is converted to the active form, methimazole. Methimazole prevents thyroid peroxidase enzyme from iodinating and coupling the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 (thyroxine).

Toxic multinodular goiter (TMNG), also known as multinodular toxic goiter (MNTG), is an active multinodular goiter associated with hyperthyroidism.
Thyroid storm is a rare but severe and life-threatening complication of hyperthyroidism. It occurs when an overactive thyroid leads to hypermetabolism, which can cause death from cardiac arrest or multiple organ failure.
Goitrogens are substances that disrupt the production of thyroid hormones. This triggers the pituitary to release thyroid-stimulating hormone (TSH), which then promotes the growth of thyroid tissue, eventually leading to goiter.

Reverse triiodothyronine (3,3′,5′-triiodothyronine, reverse T3, or rT3) is an isomer of triiodothyronine (3,5,3′ triiodothyronine, T3).
An antithyroid agent is a hormone inhibitor acting upon thyroid hormones.
Euthyroid sick syndrome (ESS) is a state of adaptation or dysregulation of thyrotropic feedback control wherein the levels of T3 and/or T4 are abnormal, but the thyroid gland does not appear to be dysfunctional. This condition may result from allostatic responses of hypothalamus-pituitary-thyroid feedback control, dyshomeostatic disorders, drug interferences, and impaired assay characteristics in critical illness.

Thyroid hormones are any hormones produced and released by the thyroid gland, namely triiodothyronine (T3) and thyroxine (T4). They are tyrosine-based hormones that are primarily responsible for regulation of metabolism. T3 and T4 are partially composed of iodine, derived from food. A deficiency of iodine leads to decreased production of T3 and T4, enlarges the thyroid tissue and will cause the disease known as simple goitre.

Iopanoic acid is an iodine-containing radiocontrast medium used in cholecystography. Both iopanoic acid and ipodate sodium are potent inhibitors of thyroid hormone release from thyroid gland, as well as of peripheral conversion of thyroxine (T4) to triiodothyronine (T3). These compounds inhibit 5'deiodinase (5'DID-1 and 5'DID-2) enzymes, which catalyse T4-T3 conversion in the thyroid cell, liver, kidney, skeletal muscle, heart, brain, pituitary. This accounts for the dramatic improvement in both subjective and objective symptoms of hyperthyroidism, particularly when they are used as an adjunctive therapy with thioamides (propylthiouracil, carbimazole). They can be used in the treatment of patients with severe thyrotoxicosis (thyroid storm) and significant morbidity (e.g., myocardial infarction, or stroke) for rapid control of elevated plasma triiodothyronine concentrations. The use of iopanoic acid for treatment of thyrotoxicosis has been discontinued in the United States.
Thyroid disease in pregnancy can affect the health of the mother as well as the child before and after delivery. Thyroid disorders are prevalent in women of child-bearing age and for this reason commonly present as a pre-existing disease in pregnancy, or after childbirth. Uncorrected thyroid dysfunction in pregnancy has adverse effects on fetal and maternal well-being. The deleterious effects of thyroid dysfunction can also extend beyond pregnancy and delivery to affect neurointellectual development in the early life of the child. Due to an increase in thyroxine binding globulin, an increase in placental type 3 deioidinase and the placental transfer of maternal thyroxine to the fetus, the demand for thyroid hormones is increased during pregnancy. The necessary increase in thyroid hormone production is facilitated by high human chorionic gonadotropin (hCG) concentrations, which bind the TSH receptor and stimulate the maternal thyroid to increase maternal thyroid hormone concentrations by roughly 50%. If the necessary increase in thyroid function cannot be met, this may cause a previously unnoticed (mild) thyroid disorder to worsen and become evident as gestational thyroid disease. Currently, there is not enough evidence to suggest that screening for thyroid dysfunction is beneficial, especially since treatment thyroid hormone supplementation may come with a risk of overtreatment. After women give birth, about 5% develop postpartum thyroiditis which can occur up to nine months afterwards. This is characterized by a short period of hyperthyroidism followed by a period of hypothyroidism; 20–40% remain permanently hypothyroid.

















