Related Research Articles
International Atomic Time is a high-precision atomic coordinate time standard based on the notional passage of proper time on Earth's geoid. TAI is a weighted average of the time kept by over 450 atomic clocks in over 80 national laboratories worldwide. It is a continuous scale of time, without leap seconds, and it is the principal realisation of Terrestrial Time. It is the basis for Coordinated Universal Time (UTC), which is used for civil timekeeping all over the Earth's surface and which has leap seconds.

The kilogram is the base unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially. It means 'one thousand grams'.

The metre is the base unit of length in the International System of Units (SI). Since 2019 the metre has been defined as the length of the path travelled by light in vacuum during a time interval of 1/299792458 of a second, where the second is defined by a hyperfine transition frequency of caesium.

Measurement is the quantification of attributes of an object or event, which can be used to compare with other objects or events. In other words, measurement is a process of determining how large or small a physical quantity is as compared to a basic reference quantity of the same kind. The scope and application of measurement are dependent on the context and discipline. In natural sciences and engineering, measurements do not apply to nominal properties of objects or events, which is consistent with the guidelines of the International vocabulary of metrology published by the International Bureau of Weights and Measures. However, in other fields such as statistics as well as the social and behavioural sciences, measurements can have multiple levels, which would include nominal, ordinal, interval and ratio scales.
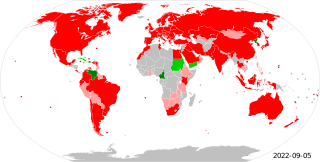
The Metre Convention, also known as the Treaty of the Metre, is an international treaty that was signed in Paris on 20 May 1875 by representatives of 17 nations: Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, Germany, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States of America, and Venezuela.

The International System of Units, internationally known by the abbreviation SI, is the modern form of the metric system and the world's most widely used system of measurement. Coordinated by the International Bureau of Weights and Measures it is the only system of measurement with an official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce.

The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived. The units and their physical quantities are the second for time, the metre for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity. The SI base units are a fundamental part of modern metrology, and thus part of the foundation of modern science and technology.

The second is the unit of time in the International System of Units (SI), historically defined as 1⁄86400 of a day – this factor derived from the division of the day first into 24 hours, then to 60 minutes and finally to 60 seconds each. "Minute" comes from the Latin pars minuta prima, meaning "first small part", and "second" comes from the pars minuta secunda, "second small part".
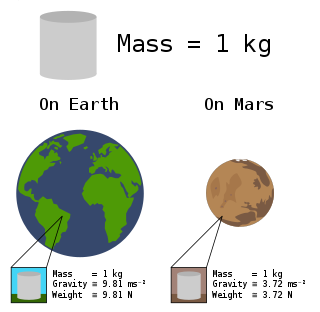
In science and engineering, the weight of an object, is the force acting on the object due to acceleration or gravity.
A time standard is a specification for measuring time: either the rate at which time passes or points in time or both. In modern times, several time specifications have been officially recognized as standards, where formerly they were matters of custom and practice. An example of a kind of time standard can be a time scale, specifying a method for measuring divisions of time. A standard for civil time can specify both time intervals and time-of-day.

The metric system is a decimal-based system of measurement. The current international standard for the metric system is the International System of Units, in which all units can be expressed in terms of seven base units: the metre, kilogram, second, ampere, kelvin, mole, and candela.

Metrology is the scientific study of measurement. It establishes a common understanding of units, crucial in linking human activities. Modern metrology has its roots in the French Revolution's political motivation to standardise units in France when a length standard taken from a natural source was proposed. This led to the creation of the decimal-based metric system in 1795, establishing a set of standards for other types of measurements. Several other countries adopted the metric system between 1795 and 1875; to ensure conformity between the countries, the Bureau International des Poids et Mesures (BIPM) was established by the Metre Convention. This has evolved into the International System of Units (SI) as a result of a resolution at the 11th General Conference on Weights and Measures (CGPM) in 1960.
The standard acceleration of gravity or standard acceleration of free fall, often called simply standard gravity and denoted by ɡ0 or ɡn, is the nominal gravitational acceleration of an object in a vacuum near the surface of the Earth. It is a constant defined by standard as 9.80665 m/s2. This value was established by the 3rd General Conference on Weights and Measures and used to define the standard weight of an object as the product of its mass and this nominal acceleration. The acceleration of a body near the surface of the Earth is due to the combined effects of gravity and centrifugal acceleration from the rotation of the Earth ; the total is about 0.5% greater at the poles than at the Equator.

The International System of Quantities (ISQ) is a standard system of quantities used in physics and in modern science in general. It includes basic quantities such as length and mass and the relationships between those quantities. This system underlies the International System of Units (SI) but does not itself determine the units of measurement used for the quantities.

A unit of measurement, or unit of measure, is a definite magnitude of a quantity, defined and adopted by convention or by law, that is used as a standard for measurement of the same kind of quantity. Any other quantity of that kind can be expressed as a multiple of the unit of measurement.

An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second:
The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.

In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artifacts such as the standard kilogram. Effective 20 May 2019, the 144th anniversary of the Metre Convention, the kilogram, ampere, kelvin, and mole are now defined by setting exact numerical values, when expressed in SI units, for the Planck constant, the elementary electric charge, the Boltzmann constant, and the Avogadro constant, respectively. The second, metre, and candela had previously been redefined using physical constants. The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements. In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes, which the International Committee for Weights and Measures (CIPM) had proposed earlier that year after determining that previously agreed conditions for the change had been met. These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.

The history of the metric system began during the Age of Enlightenment with measures of length and weight derived from nature, along with their decimal multiples and fractions. The system became the standard of France and Europe within half a century. Other measures with unity ratios were added, and the system went on to be adopted across the world.

The following outline is provided as an overview of and topical guide to the metric system:
Time metrology or time and frequency metrology is the application of metrology for time keeping, including frequency stability. Its main tasks are the realization of the second as the SI unit of measurement for time and the establishment of time standards and frequency standards as well as their dissemination.
References
- 1 2 "Realise" . Oxford English Dictionary (Online ed.). Oxford University Press.(Subscription or participating institution membership required.)
- ↑ International Bureau of Weights and Measures (2012). "Practical realization of the definitions of some important units". p. 46. Retrieved 23 April 2013.
- ↑ International vocabulary of metrology—Basic and general concepts and associated terms (VIM) (PDF) (3rd ed.). International Bureau of Weights and Measures on behalf of the Joint Committee for Guides in Metrology. 2012. Retrieved 26 April 2013.
- ↑ Quinn, T. J. (2003). "Practical realisation of the definition of the metre, including recommended radiations of other optical frequency standards (2001)" (PDF). Metrologia. 40: 103–133. Bibcode:2003Metro..40..103Q. doi:10.1088/0026-1394/40/2/316 . Retrieved 6 December 2013.